DNAJB4
Gene Information
- Official Symbol: DNAJB4
- Official Name: DnaJ heat shock protein family (Hsp40) member B4
- Aliases and Previous Symbols: N/A
- Entrez ID: 11080
- UniProt: Q9UDY4
- Interactions: BioGRID
- PubMed articles: Open PubMed
- OMIM: Open OMIM
Function Summary
- Entrez Summary: N/A
- UniProt Summary: Probable chaperone. Stimulates ATP hydrolysis and the folding of unfolded proteins mediated by HSPA1A/B (in vitro) (PubMed:24318877). {ECO:0000269|PubMed:24318877}.
CRISPR Data
Essentiality in NALM6
- Essentiality Rank: 6051
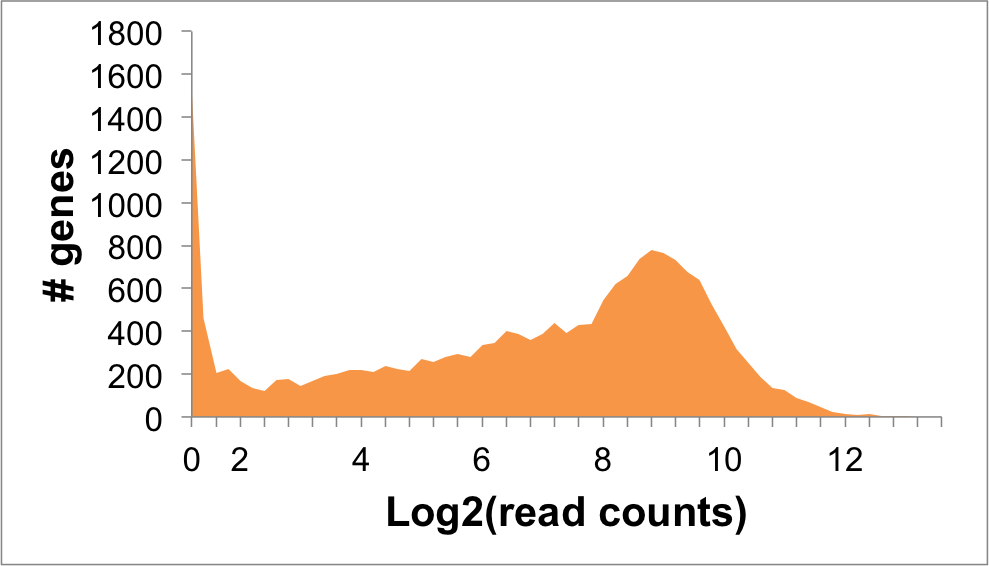
- Expression level (log2 read counts): 3.56