This is an old revision of the document!
5-Fluorouracil 2μM R00 exp1
Mechanism of Action
Thymidylate synthase inhibitor, blocks DNA replication
- Class / Subclass 1: Metabolism / Antimetabolite
- Class / Subclass 2: DNA Damage, Repair and Replication / Replication Inhibitor
Technical Notes
Compound References
- PubChem Name: 5-Fluorouracil
- Synonyms: 5-FU
- CAS #: 51-21-8
- PubChem CID: 3385
- IUPAC: 5-fluoro-1H-pyrimidine-2,4-dione
- INCHI Name: InChI=1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
- INCHI Key: GHASVSINZRGABV-UHFFFAOYSA-N
- Molecular Weight: 130.08
- Canonical SMILES: C1=C(C(=O)NC(=O)N1)F
- Isomeric SMILES: NA
- Molecular Formula: C4H3FN2O2
Compound Supplier
- Supplier Name: Sigma-Aldrich
- Catalog #: F6627
- Lot #: 102K0970
Compound Characterization
- LCMS: Tr 0.14 min, m/z 129- [M-H]-
Dose Response Curve
- Platform ID: 5-FU
- Min: -8.9515; Max: 61.1847

| IC | Concentration (µM) |
|---|---|
| IC10 | NA |
| IC20 | 12.7820 |
| IC30 | 22.9250 |
| IC40 | 37.0071 |
| IC50 | 57.4305 |
| IC60 | NA |
| IC70 | NA |
| IC80 | NA |
| IC90 | NA |
Screen Results
- Round: 00
- Dose: 2µM
- Days of incubation: 8
- Doublings: 5.6
- Numbers of reads: 10701088
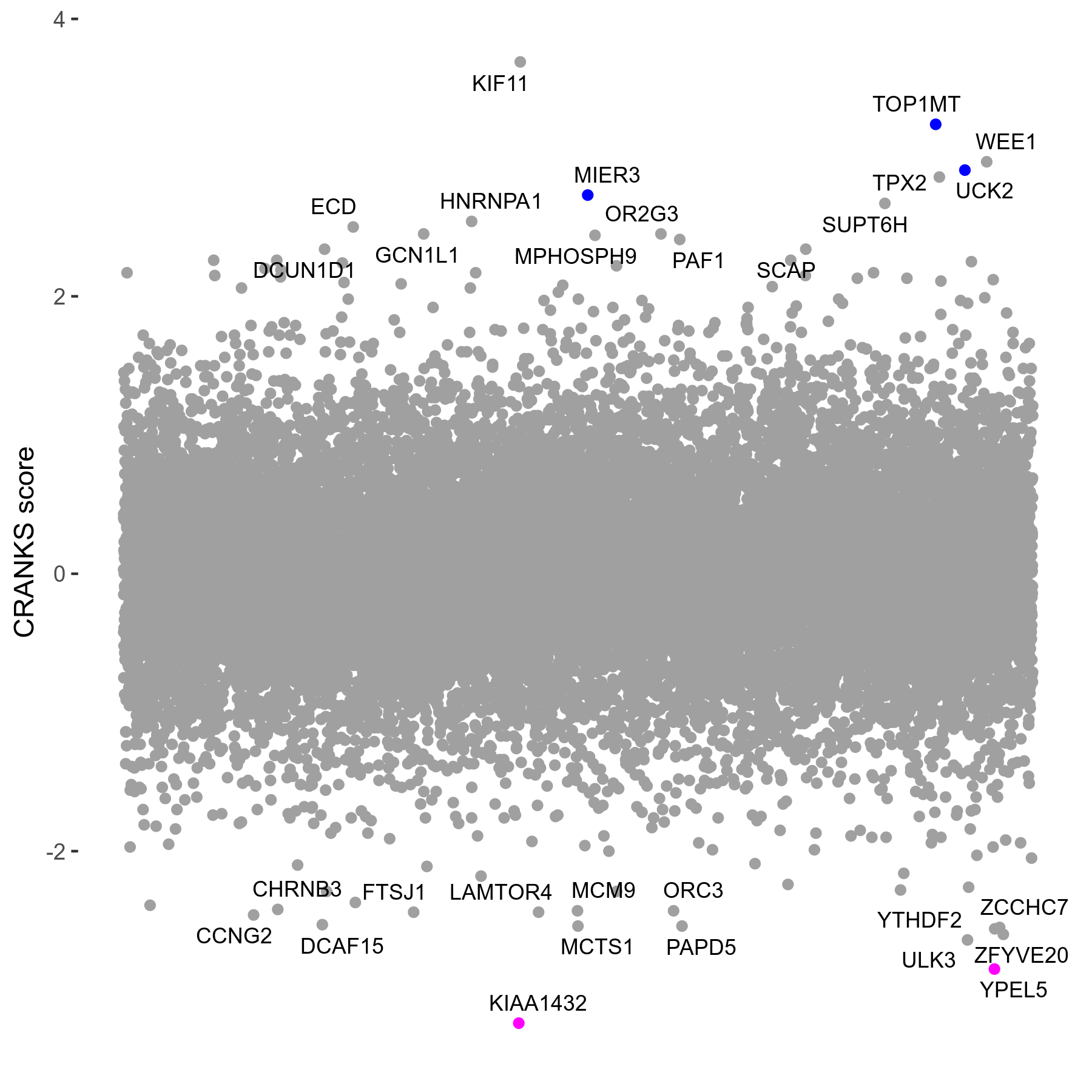
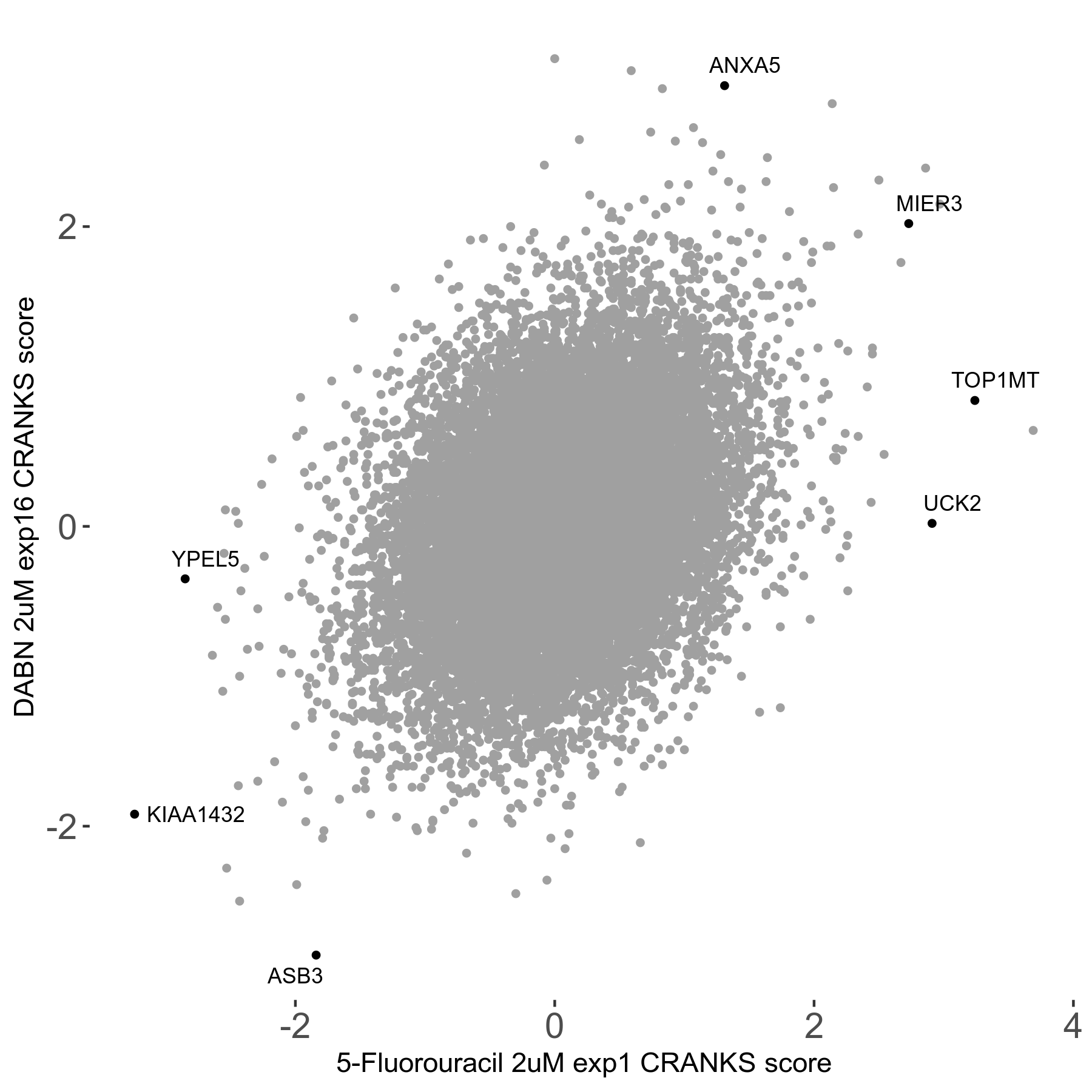
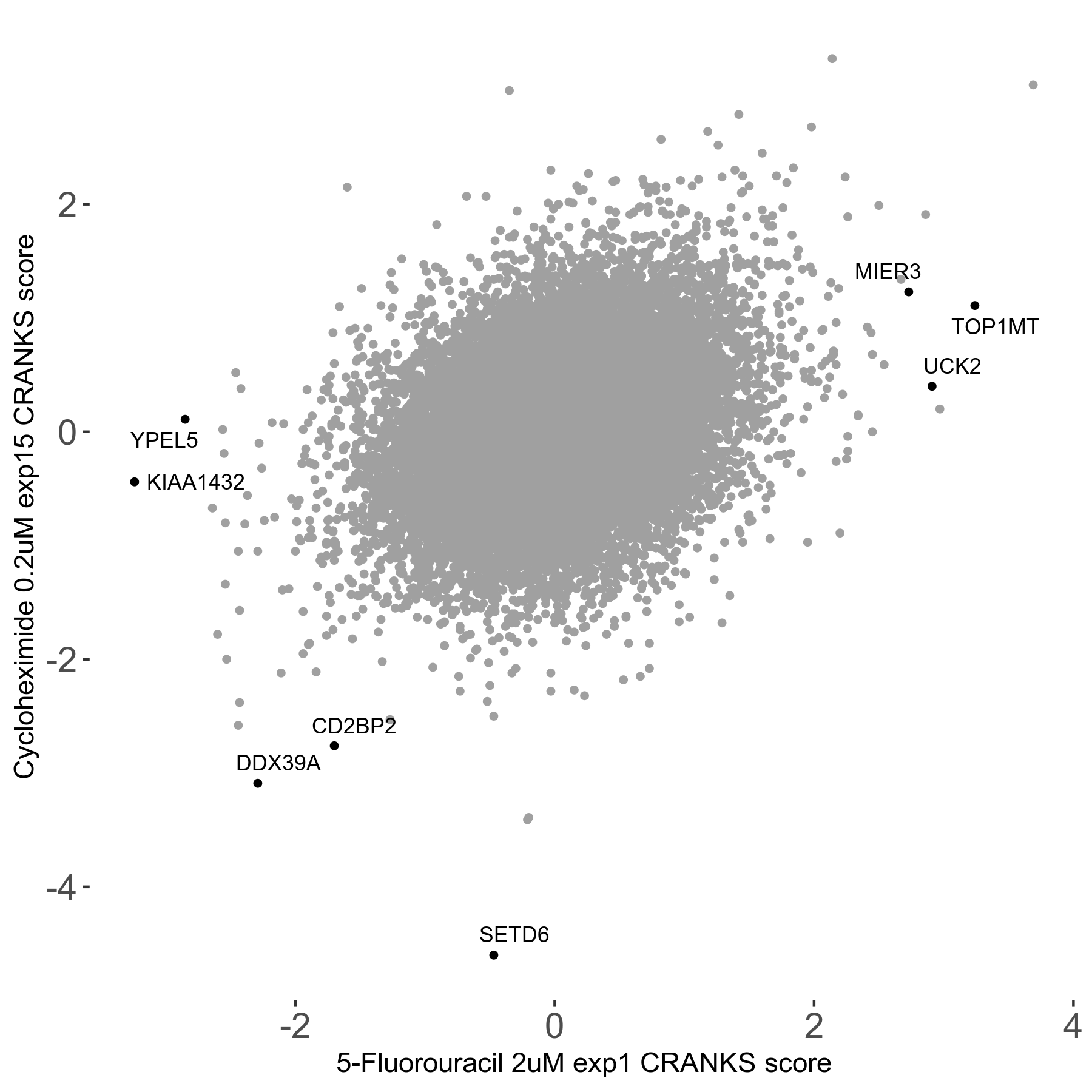
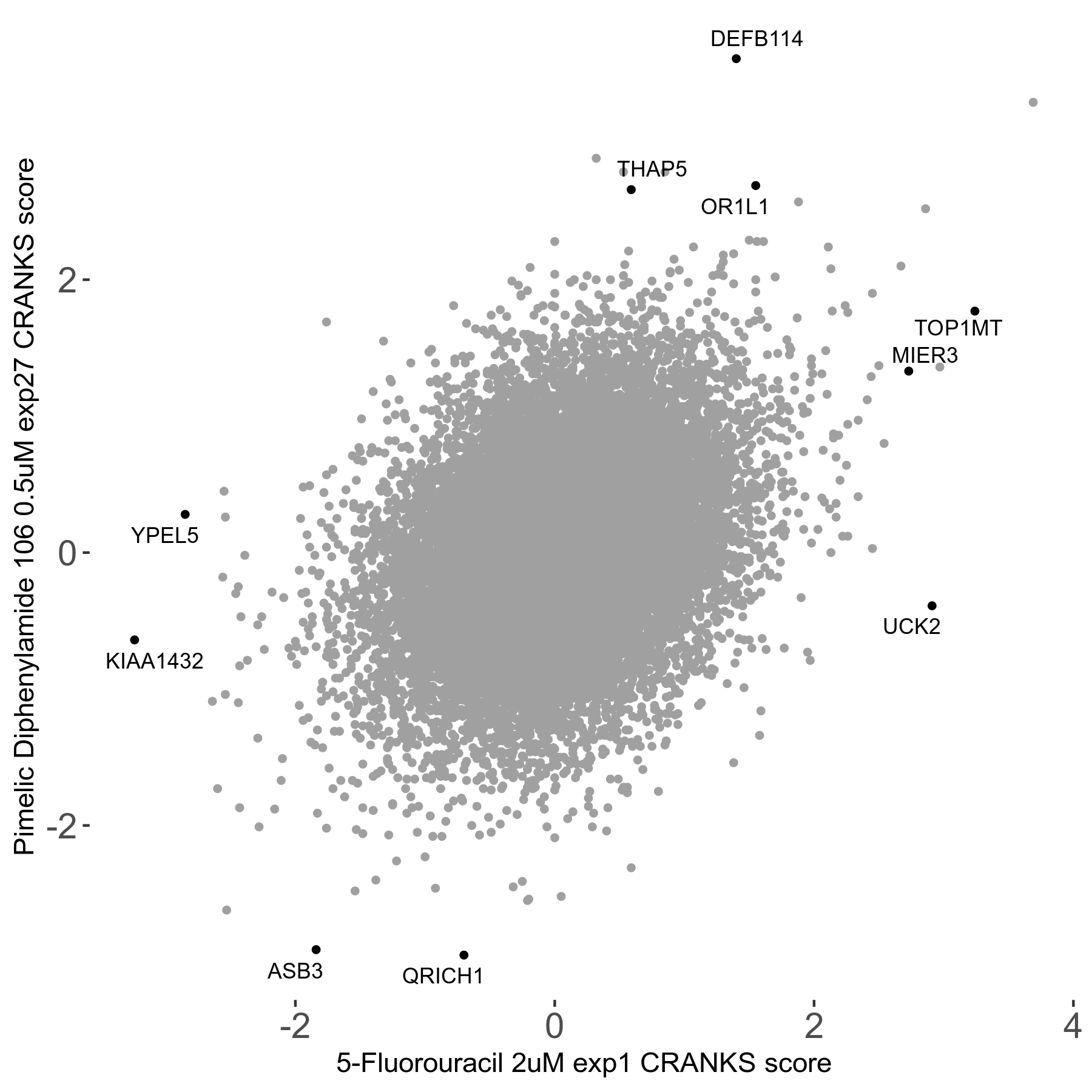
Screen Summary
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 2/3 | Scores |