BI-6727 0.001μM R01 exp42
Mechanism of Action
Inhibits PLK1 kinase, causes G2/M arrest, improved version of BI-2536
- Class / Subclass 1: Cell Cycle / Mitotic Inhibitor
- Class / Subclass 2: Signal Transduction / Kinase Inhibitor
Technical Notes
Compound References
- PubChem Name: Volasertib
- Synonyms: BI 6727
- CAS #: 755038-65-4
- PubChem CID: 10461508
- IUPAC: N-[4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexyl]-4-[[(7R)-7-ethyl-5-methyl-6-oxo-8-propan-2-yl-7H-pteridin-2-yl]amino]-3-methoxybenzamide
- INCHI Name: InChI=1S/C34H50N8O3/c1-6-28-33(44)39(4)29-20-35-34(38-31(29)42(28)22(2)3)37-27-14-9-24(19-30(27)45-5)32(43)36-25-10-12-26(13-11-25)41-17-15-40(16-18-41)21-23-7-8-23/h9,14,19-20,22-23,25-26,28H,6-8,10-13,15-18,21H2,1-5H3,(H,36,43)(H,35,37,38)/t25?,26?,28-/m1/s1
- INCHI Key: SXNJFOWDRLKDSF-XKHVUIRMSA-N
- Molecular Weight: 618.8
- Canonical SMILES: CCC1C(=O)N(C2=CN=C(N=C2N1C(C)C)NC3=C(C=C(C=C3)C(=O)NC4CCC(CC4)N5CCN(CC5)CC6CC6)OC)C
- Isomeric SMILES: CC[C@@H]1C(=O)N(C2=CN=C(N=C2N1C(C)C)NC3=C(C=C(C=C3)C(=O)NC4CCC(CC4)N5CCN(CC5)CC6CC6)OC)C
- Molecular Formula: C34H50N8O3
Compound Supplier
- Supplier Name: Med Chem Express
- Catalog #: HY-12137
- Lot #: N/A
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C34H50N8O3 619.40786; found 619.4097
Dose Response Curve
- Platform ID: BI6727
- Min: -7.8407; Max: 99.1028

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 0.0061 |
| IC30 | 0.0129 |
| IC40 | 0.0238 |
| IC50 | 0.0420 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 01
- Dose: 1nM
- Days of incubation: 8
- Doublings: 6.8
- Numbers of reads: 12280512
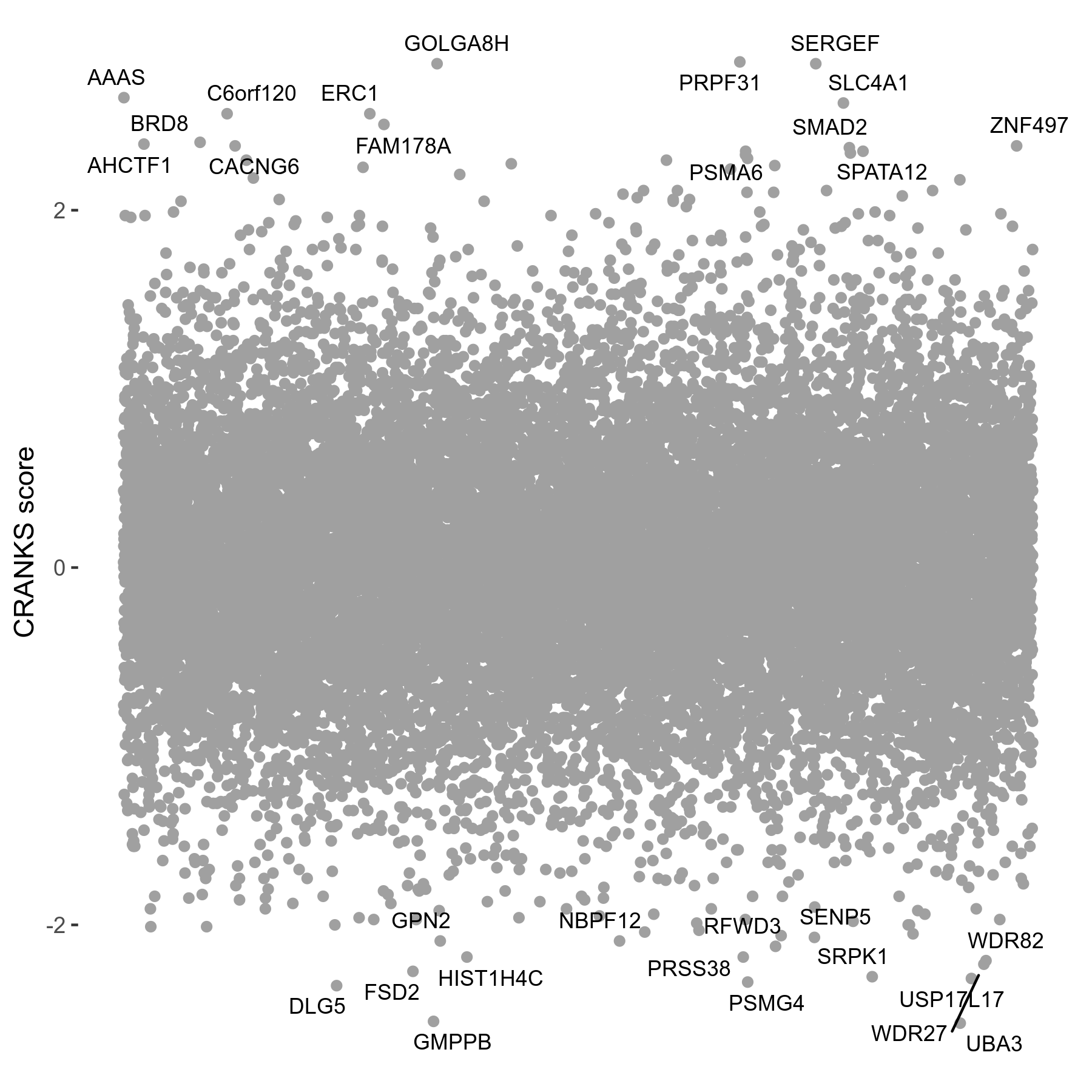
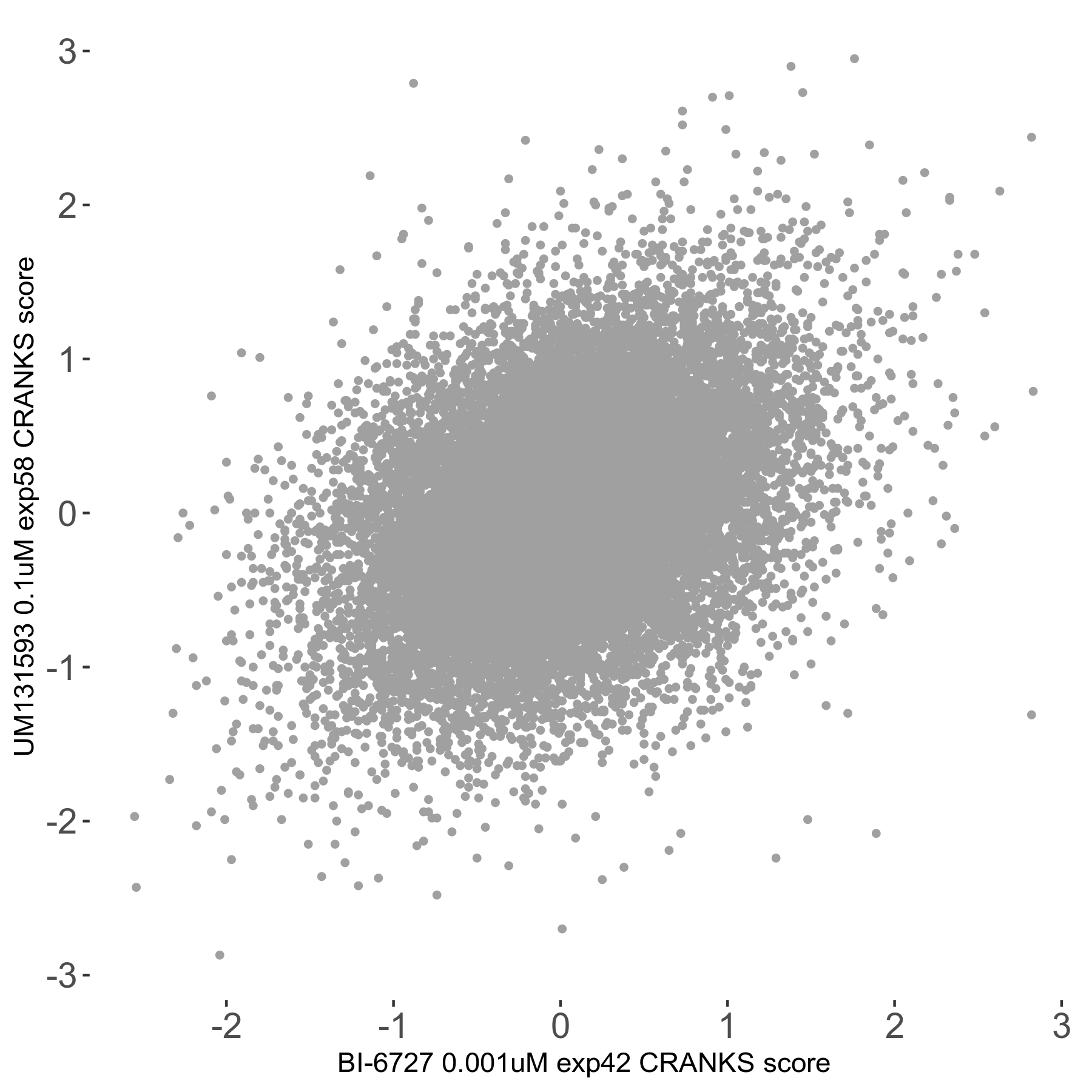
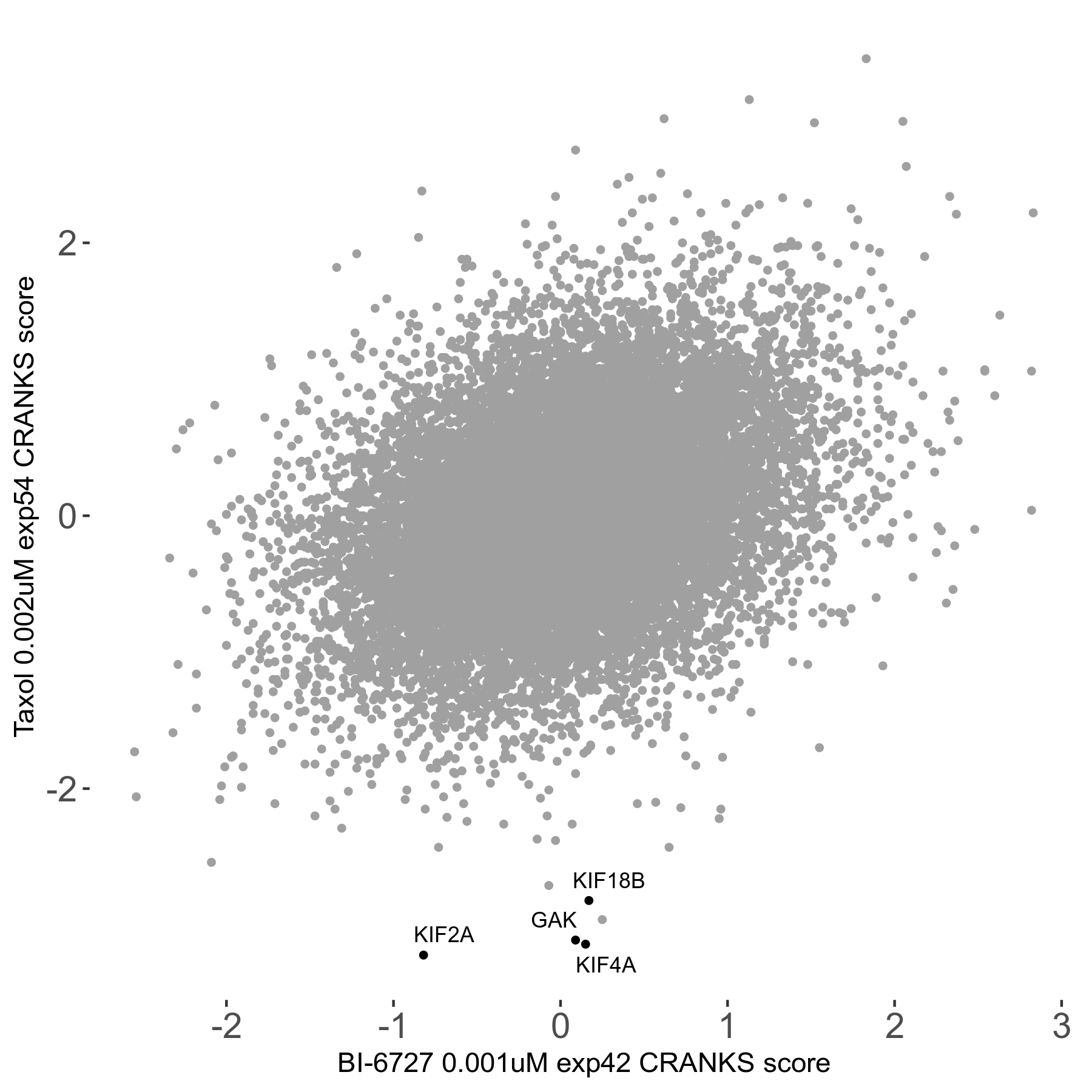
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 0/0 | Scores |