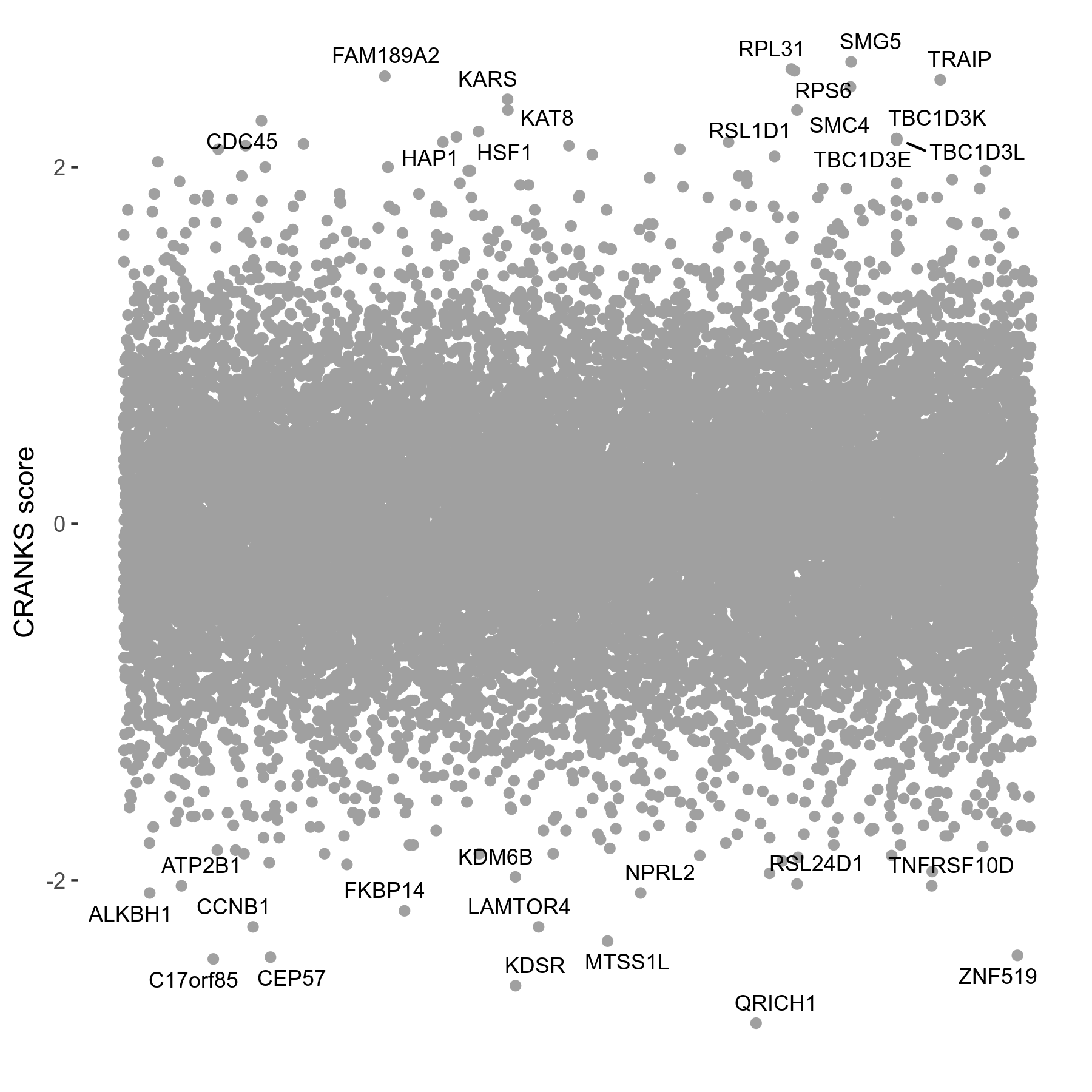
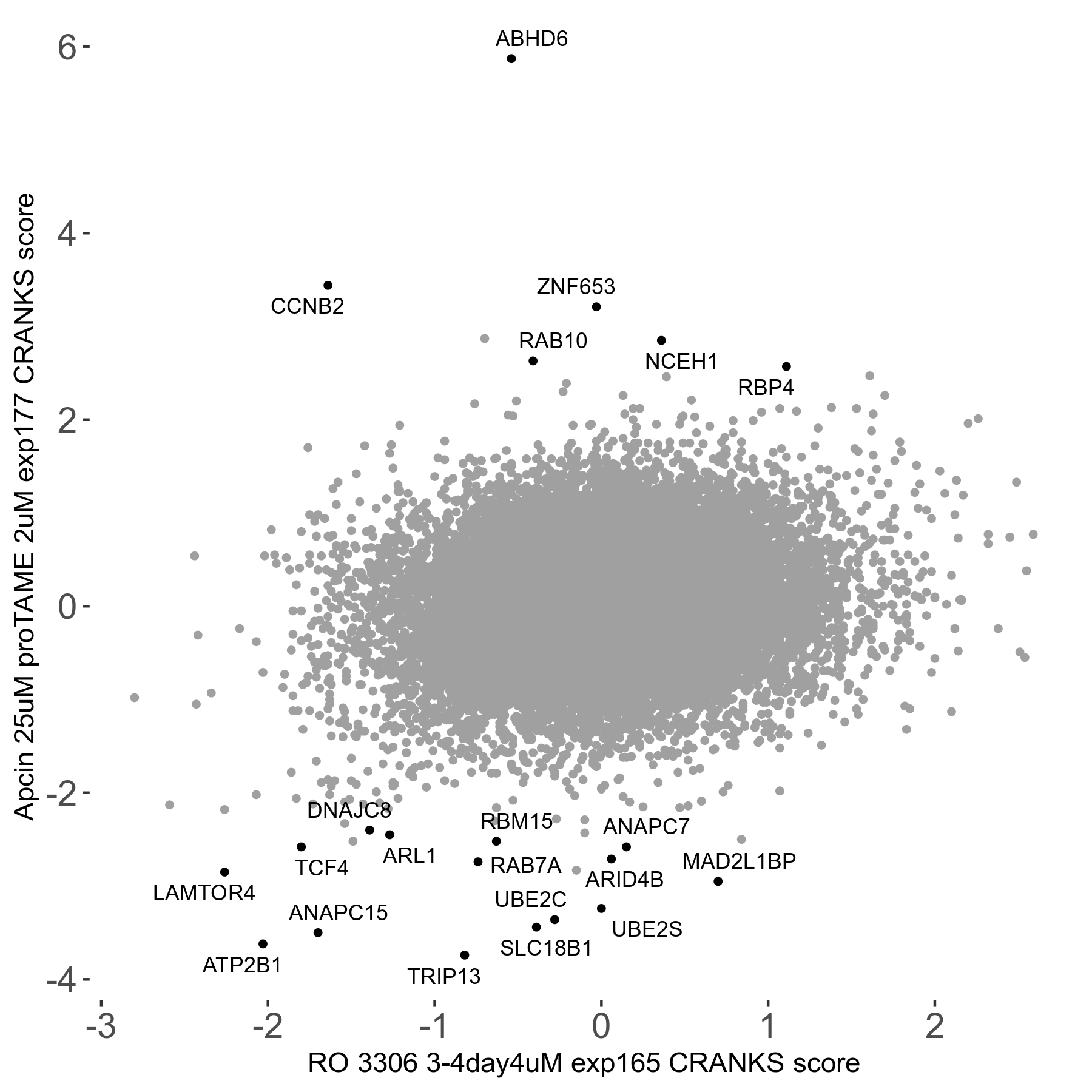
RO-3306 3 to 4μM on day4 R04 exp165
Mechanism of Action
Inhibits CDK1, ATP-competitive, causes G2/M arrest
- Class / Subclass 1: Cell Cycle / Kinase Inhibitor
Technical Notes
Compound References
- PubChem Name: (5Z)-5-(Quinolin-6-ylmethylidene)-2-[(thiophen-2-ylmethyl)amino]-1,3-thiazol-4(5H)-one
- Synonyms: N/A
- CAS #: 872573-93-8
- PubChem CID: 135400873
- IUPAC: (5Z)-5-(quinolin-6-ylmethylidene)-2-(thiophen-2-ylmethylimino)-1,3-thiazolidin-4-one
- INCHI Name: InChI=1S/C18H13N3OS2/c22-17-16(24-18(21-17)20-11-14-4-2-8-23-14)10-12-5-6-15-13(9-12)3-1-7-19-15/h1-10H,11H2,(H,20,21,22)/b16-10-
- INCHI Key: XOLMRFUGOINFDQ-YBEGLDIGSA-N
- Molecular Weight: 351.4
- Canonical SMILES: C1=CC2=C(C=CC(=C2)C=C3C(=O)NC(=NCC4=CC=CS4)S3)N=C1
- Isomeric SMILES: C1=CC2=C(C=CC(=C2)/C=C\\3/C(=O)NC(=NCC4=CC=CS4)S3)N=C1
- Molecular Formula: C18H13N3OS2
Compound Supplier
- Supplier Name: Tocris Bioscience
- Catalog #: 4181
- Lot #: N/A
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C18H13N3OS2 352.05728; found 352.05802
Dose Response Curve
- Platform ID: RO-3306
- Min: -19.9269; Max: 63.7506

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 5.6881 |
| IC30 | 7.8448 |
| IC40 | 10.2102 |
| IC50 | 13.0035 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 04
- Dose: 3-4µM
- Days of incubation: 8
- Doublings: 7.1
- Numbers of reads: 18779480
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 0/0 | Scores |