Pifithrin-alpha 20μM R07 exp390
Mechanism of Action
Claimed to inhibit p53 but has p53-independent effects, cyclic form may increase stem cell reprogramming
- Class / Subclass 1: Signal Transduction / Apoptosis Inhibitor
Technical Notes
Protein References
- PubChem Name: Pifithrin-alpha
- Synonyms: Pifithrin hydrobromide; PFTα hydrobromide
- CAS #: 63208-82-2
- PubChem CID: 9929138
- IUPAC: 2-(2-imino-4,5,6,7-tetrahydro-1,3-benzothiazol-3-yl)-1-(4-methylphenyl)ethanone;hydrobromide
- INCHI Name: InChI=1S/C16H18N2OS.BrH/c1-11-6-8-12(9-7-11)14(19)10-18-13-4-2-3-5-15(13)20-16(18)17;/h6-9,17H,2-5,10H2,1H3;1H
- INCHI Key: HAGVCKULCLQGRF-UHFFFAOYSA-N
- Molecular Weight: 367.3
- Canonical SMILES: CC1=CC=C(C=C1)C(=O)CN2C3=C(CCCC3)SC2=N.Br
- Isomeric SMILES: N/A
- Molecular Formula: C16H19BrN2OS
Protein Supplier
- Supplier Name: Sigma-Aldrich
- Catalog #: P4359
- Lot #: N/A
Protein Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C16H18N2OS 287.12126; found 287.12088
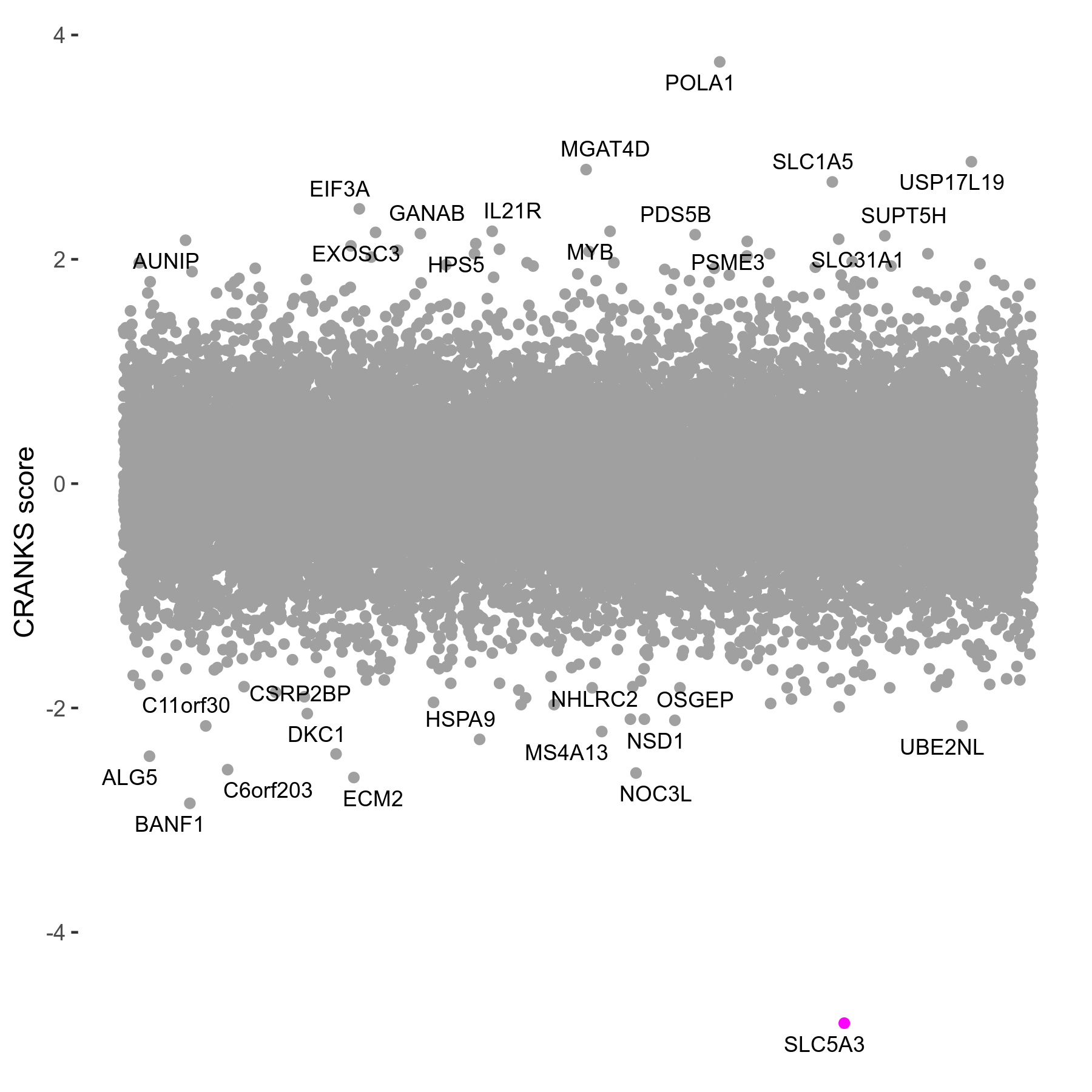
Dose Response Curve
- Platform ID: pifithrin-alpha
- Min: -5.8398; Max: 30.3674

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | N/A |
| IC30 | N/A |
| IC40 | N/A |
| IC50 | N/A |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 07
- Dose: 20µM
- Days of incubation: 8
- Doublings: 6.0
- Numbers of reads: 20313436
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 1/0 | Scores |