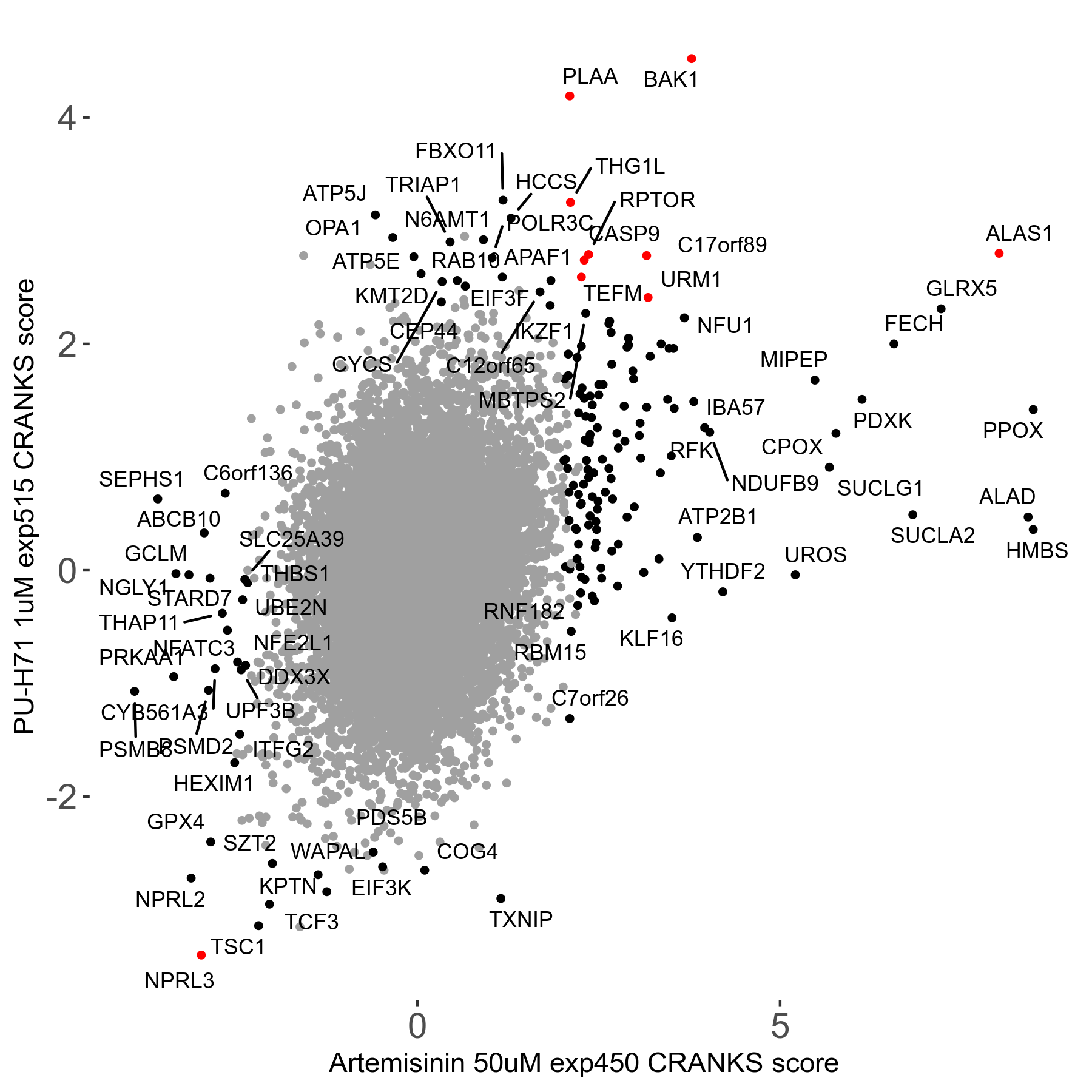
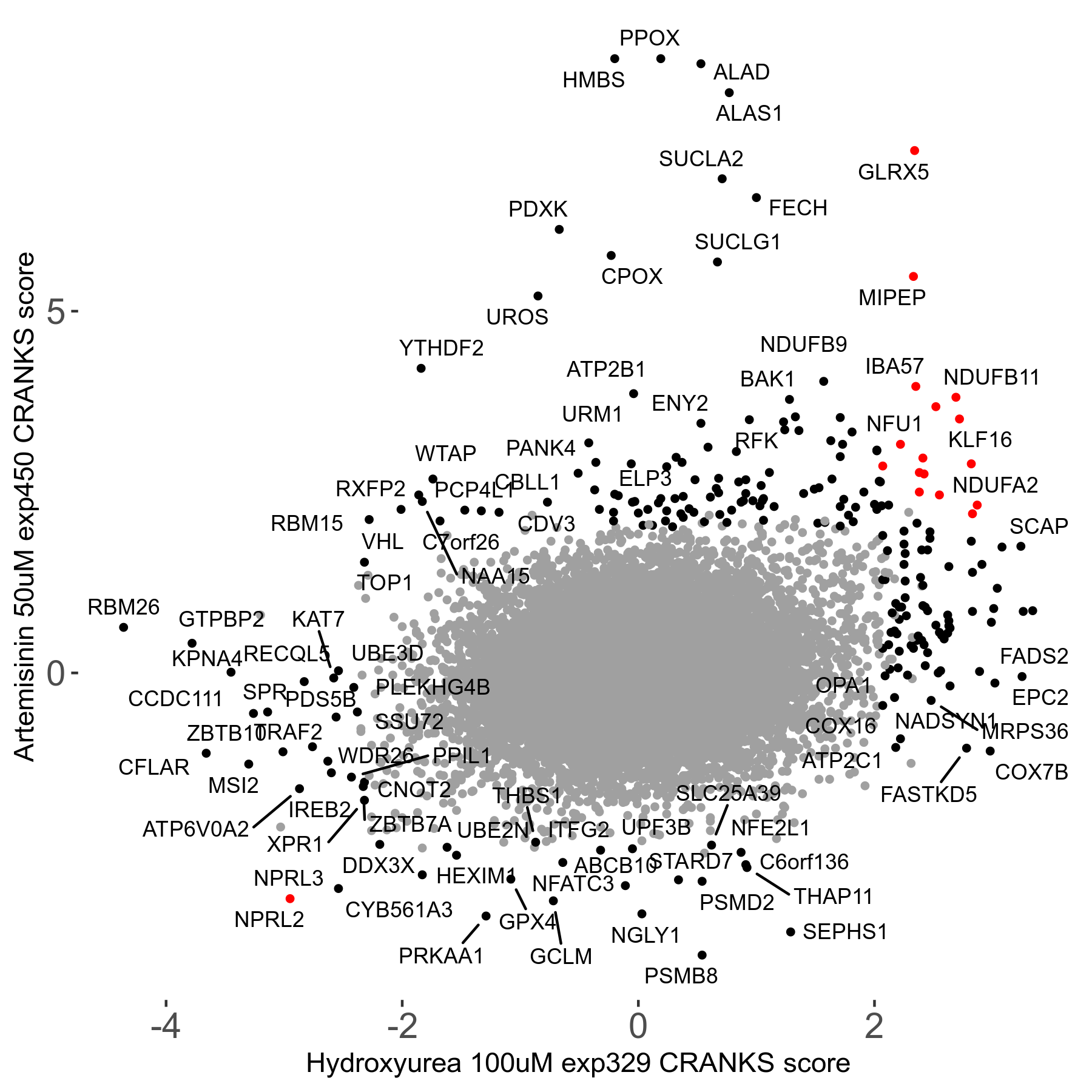
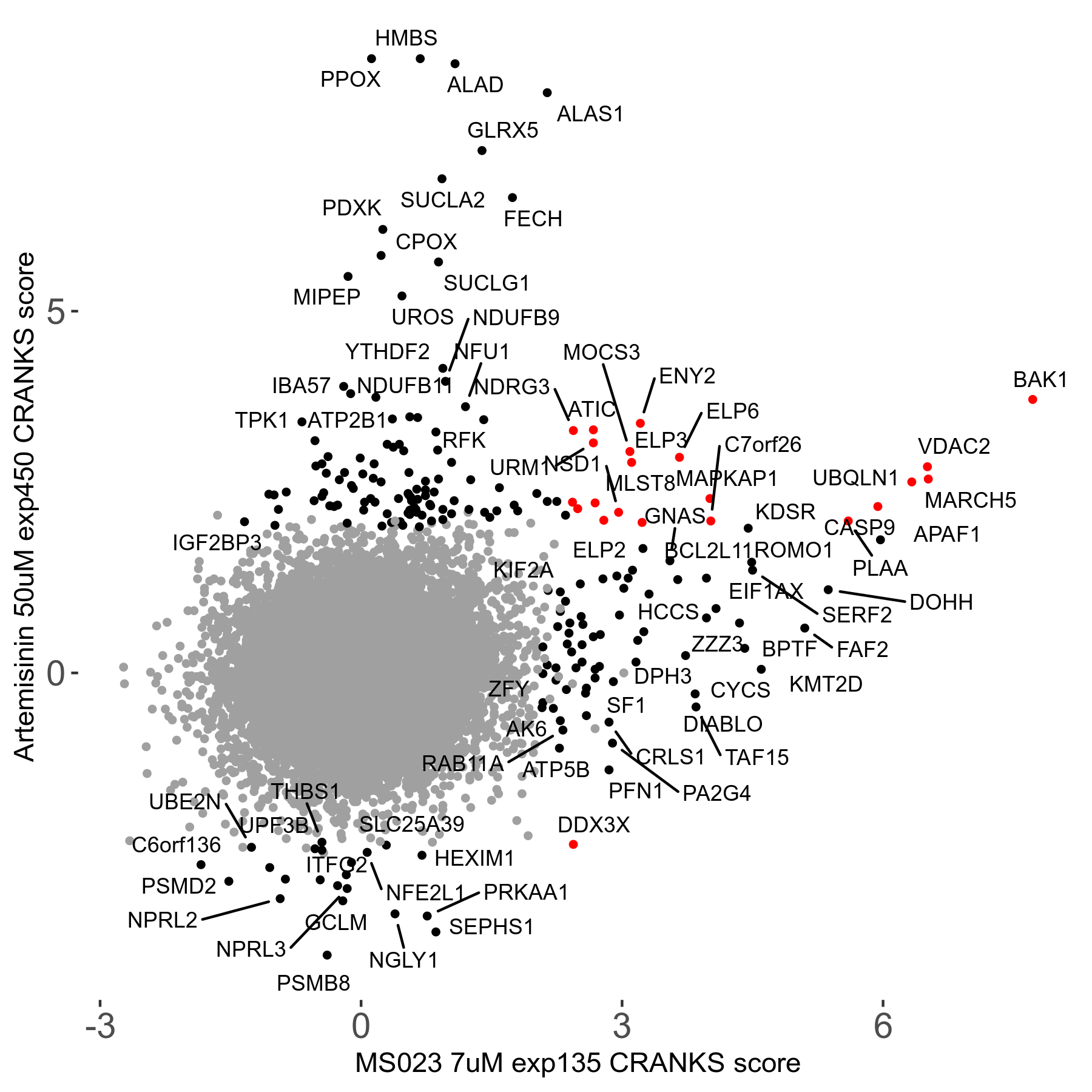
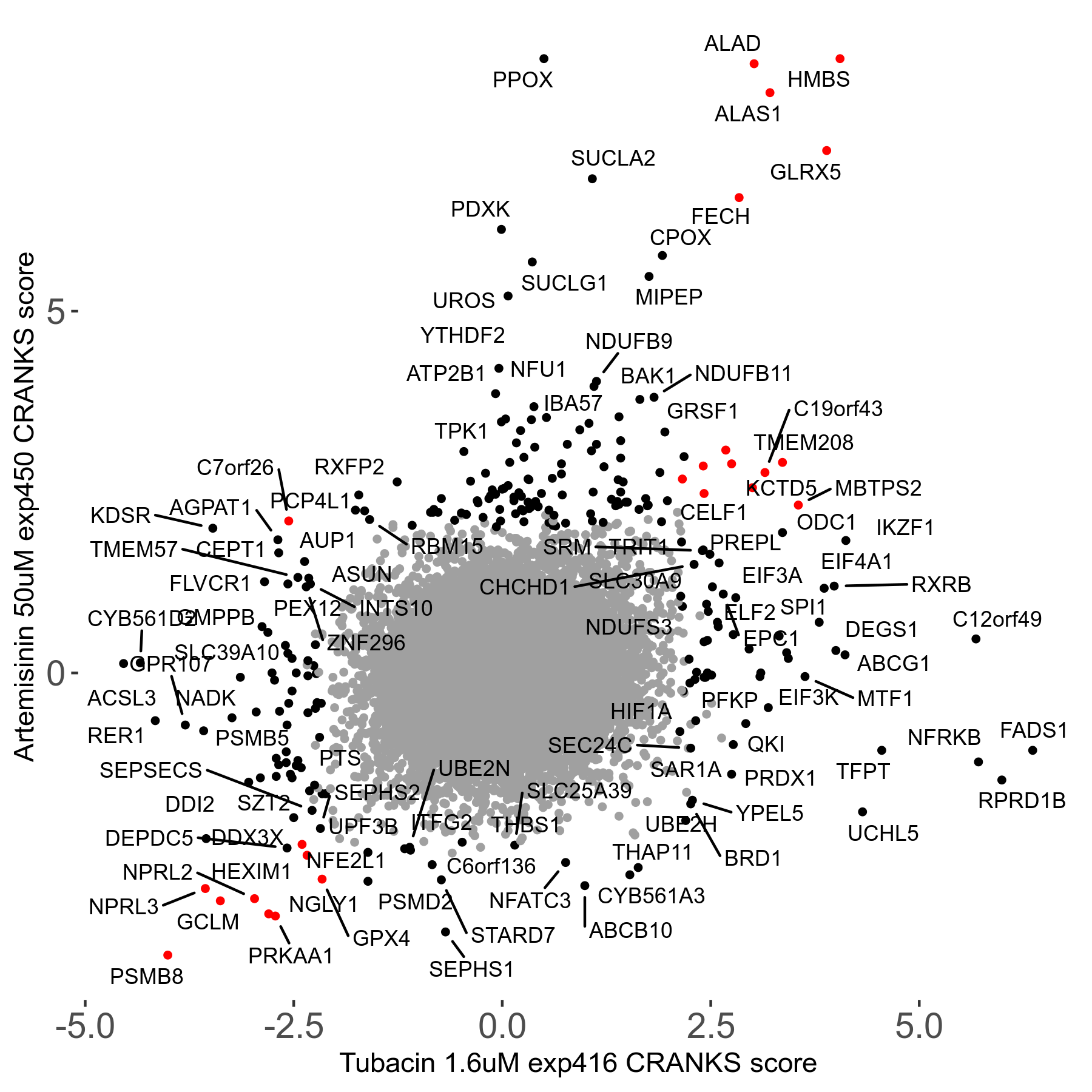
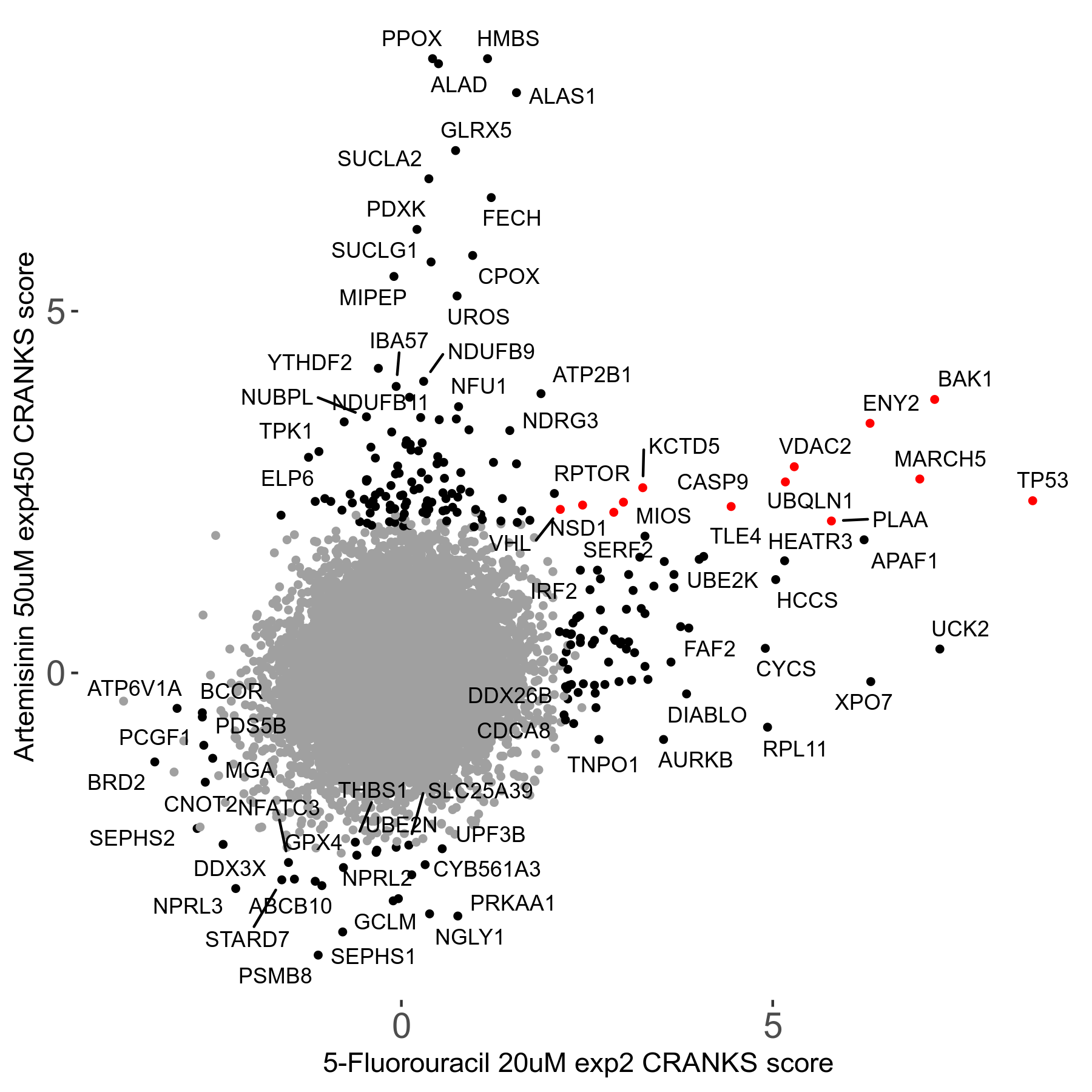
Artemisinin 50μM R08 exp450
Mechanism of Action
Sesquiterpene endoperoxide activated by heme, kills malaria parasite through oxidative damage
- Class / Subclass 1: Infectious Disease / Antimalarial
Technical Notes
Compound References
- PubChem Name: Artemisinin
- Synonyms: Qinghaosu; NSC 369397
- CAS #: 63968-64-9
- PubChem CID: 68827
- IUPAC: (1R,4S,5R,8S,9R,12S,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one
- INCHI Name: InChI=1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1
- INCHI Key: BLUAFEHZUWYNDE-NNWCWBAJSA-N
- Molecular Weight: 282.33
- Canonical SMILES: CC1CCC2C(C(=O)OC3C24C1CCC(O3)(OO4)C)C
- Isomeric SMILES: C[C@@H]1CC[C@H]2[C@H](C(=O)O[C@H]3[C@@]24[C@H]1CC[C@](O3)(OO4)C)C
- Molecular Formula: C15H22O5
Compound Supplier
- Supplier Name: Combi-Blocks
- Catalog #: QA-7358
- Lot #: B22262
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C15H22O5 283.154; found 283.15465
Dose Response Curve
- Platform ID: Artemisinin
- Min: -15.4586; Max: 33.8695

| IC | Concentration (µM) |
|---|---|
| IC10 | 7.4780 |
| IC20 | 34.7400 |
| IC30 | 96.4000 |
| IC40 | N/A |
| IC50 | N/A |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 08
- Dose: 50µM
- Days of incubation: 8
- Doublings: 1.9
- Numbers of reads: 31050357
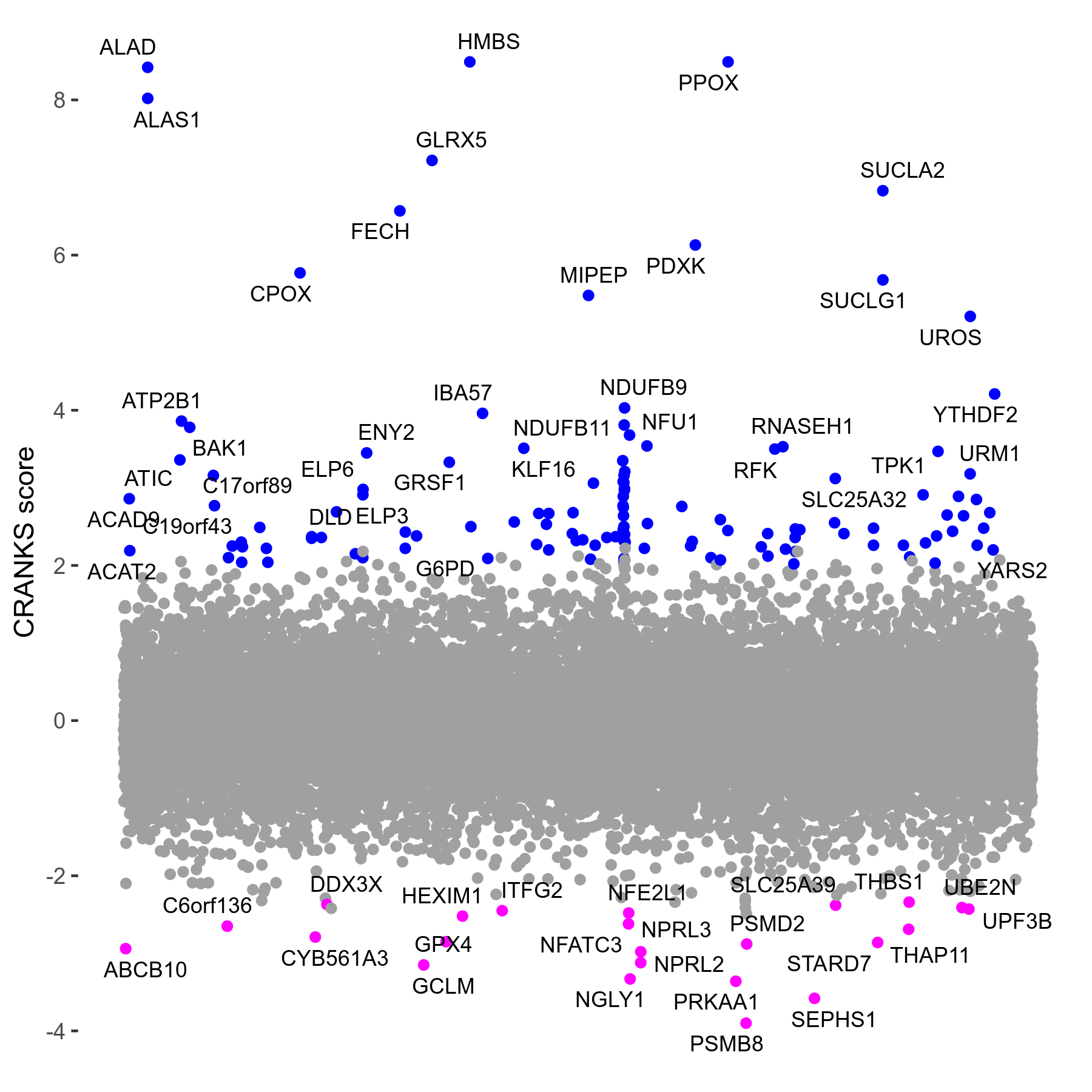
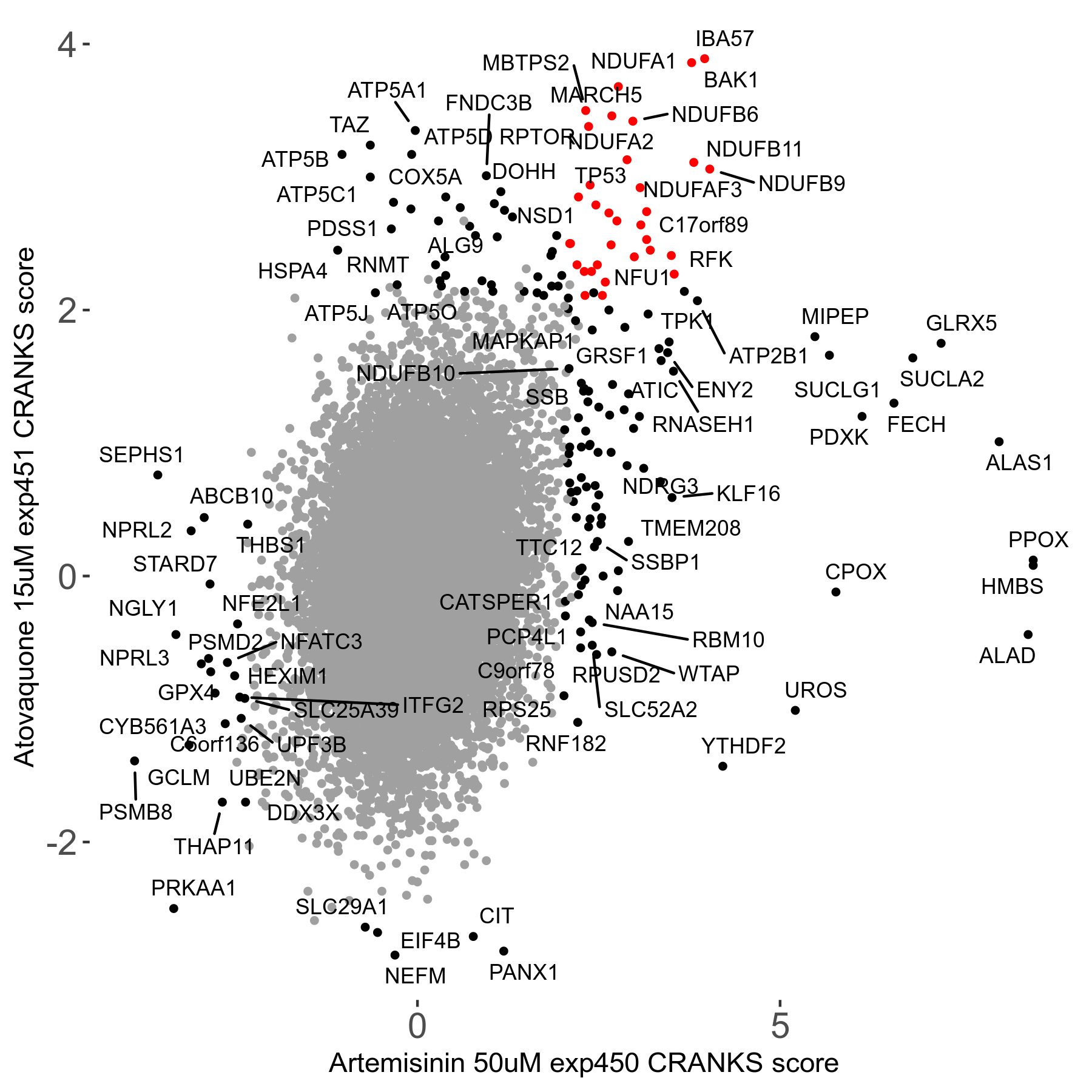
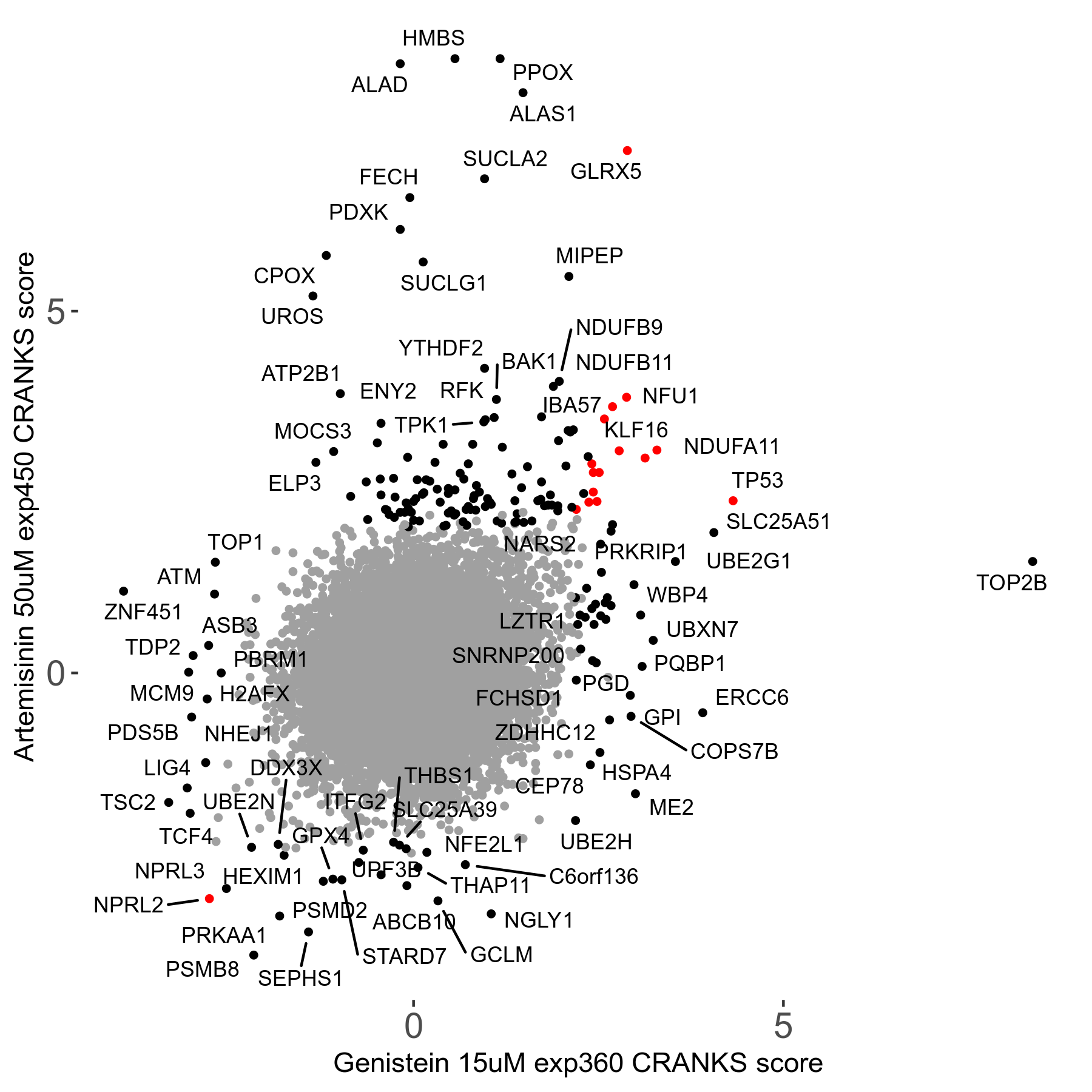
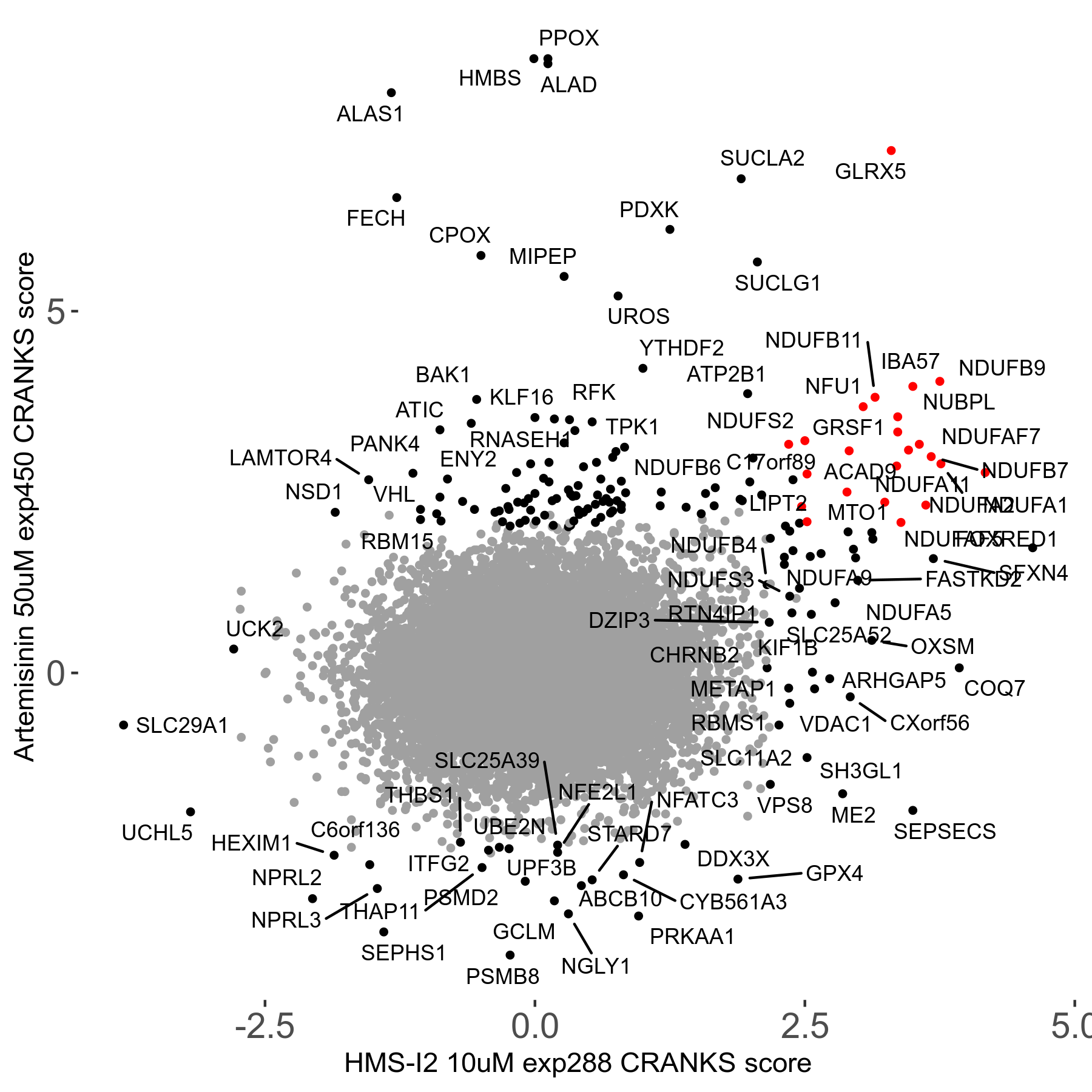
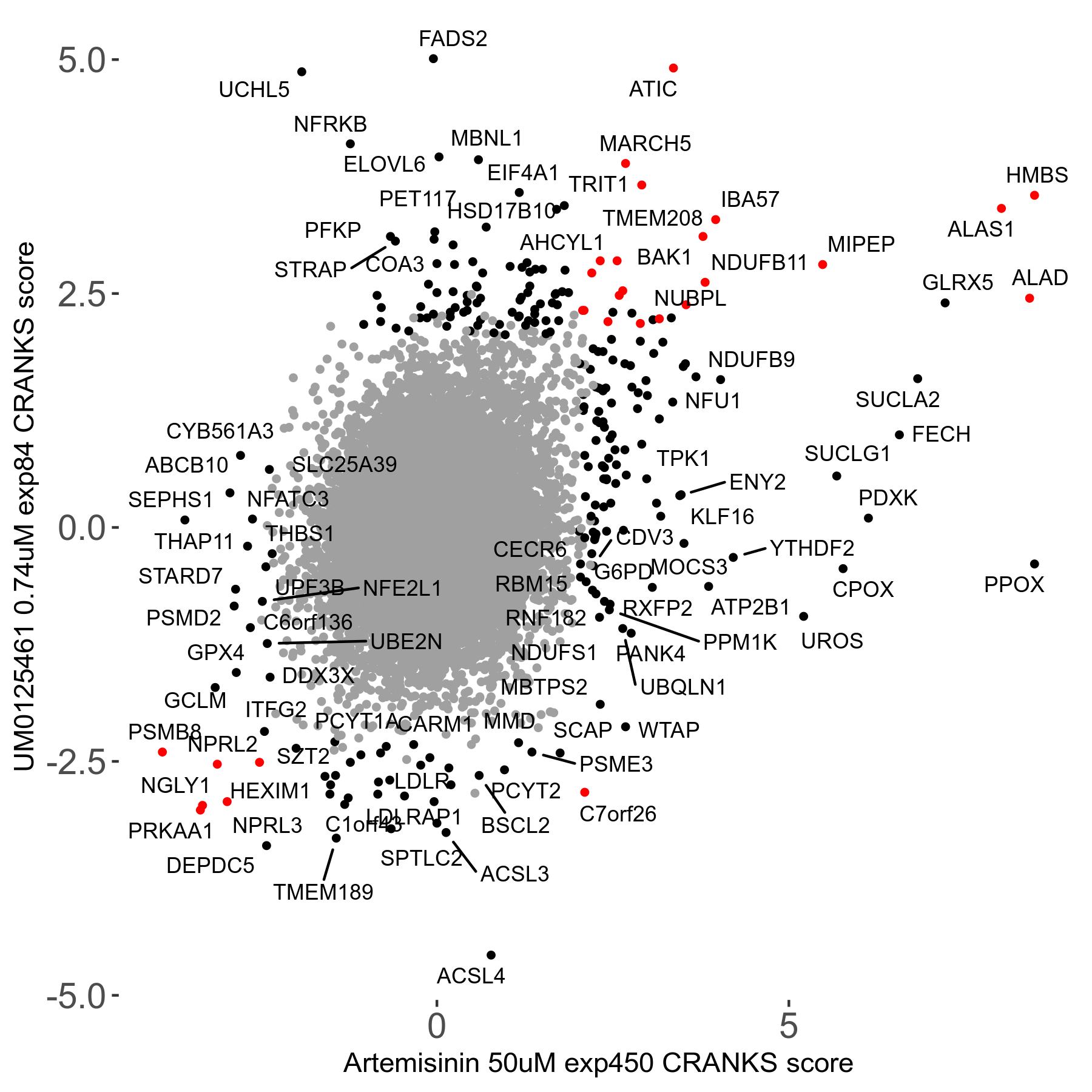
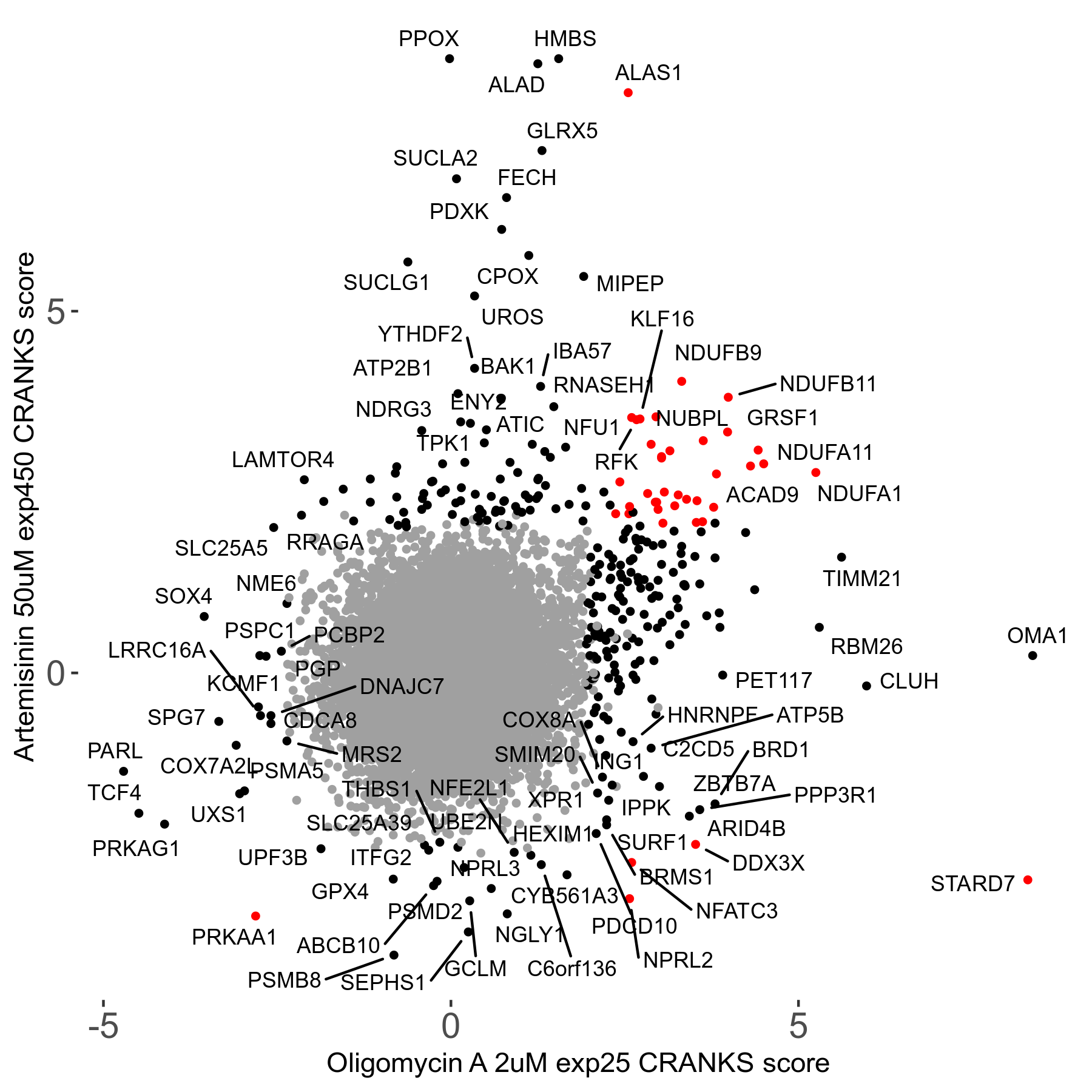
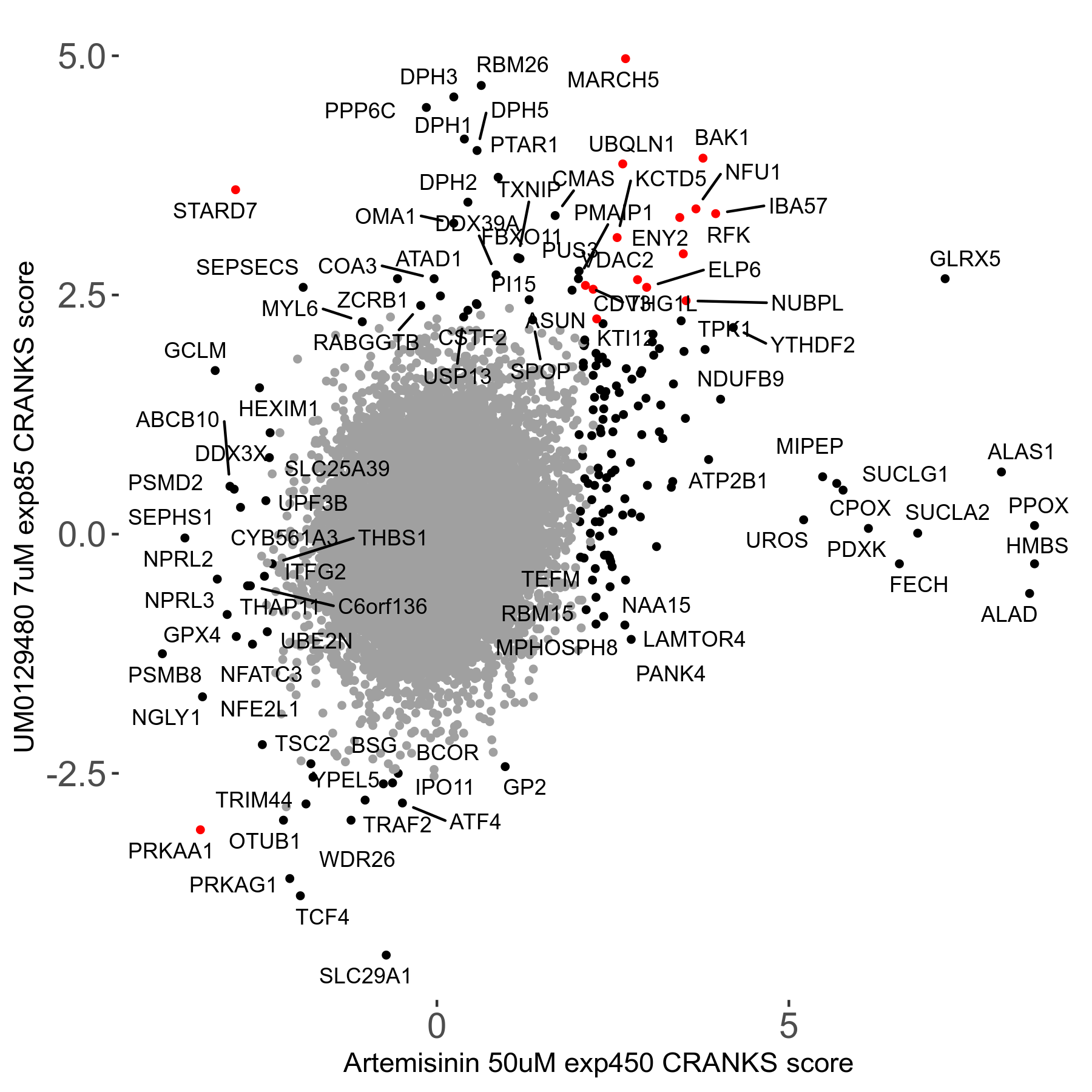
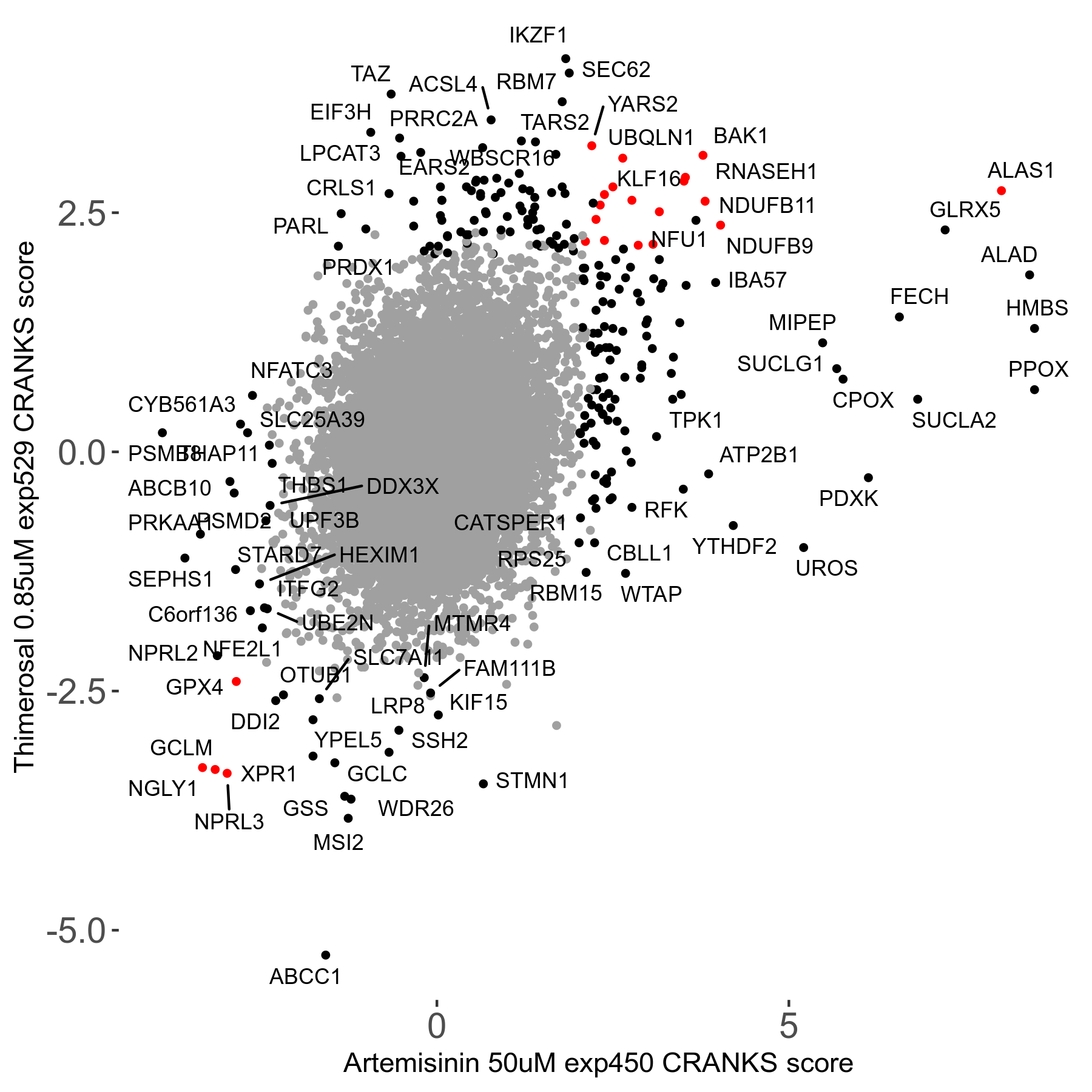
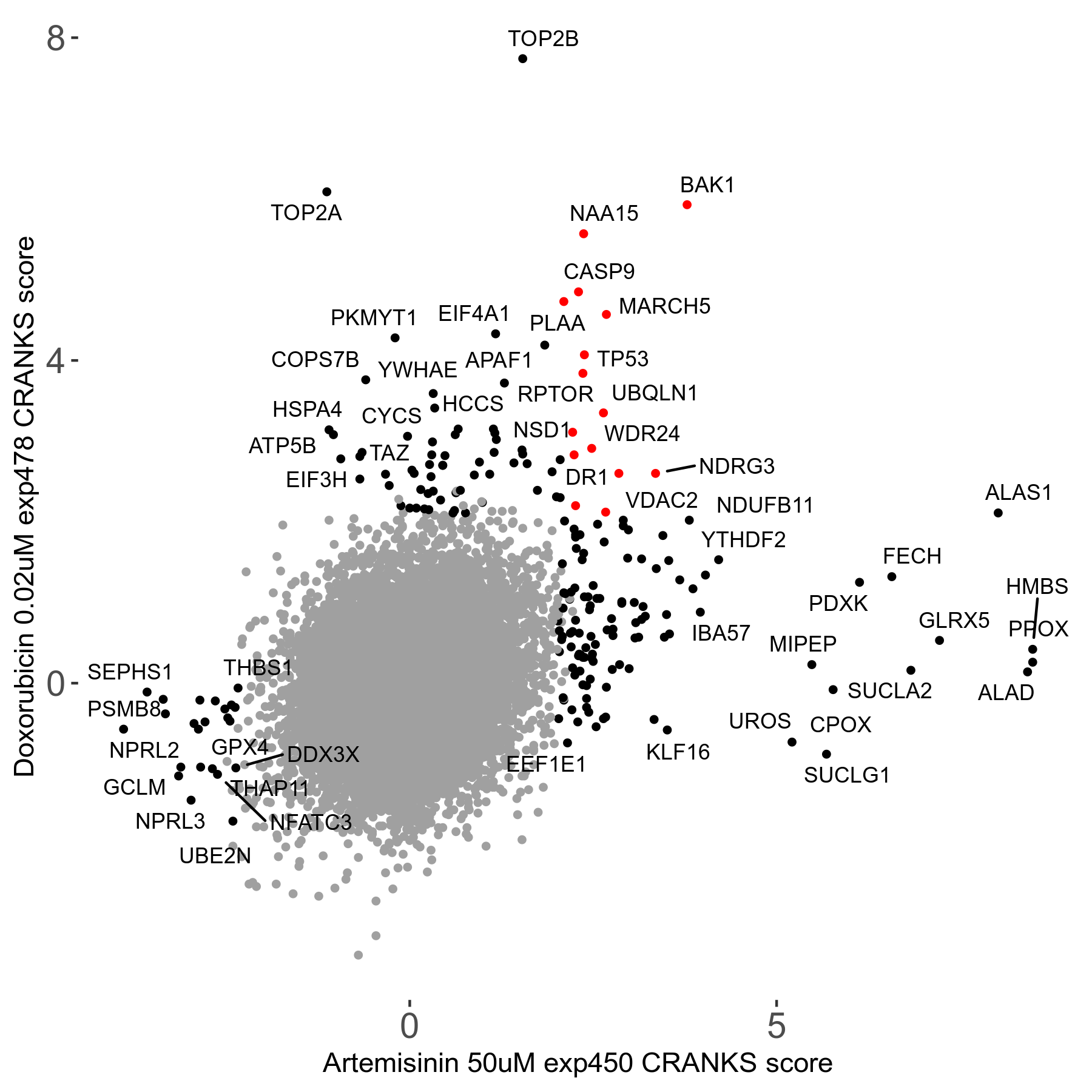
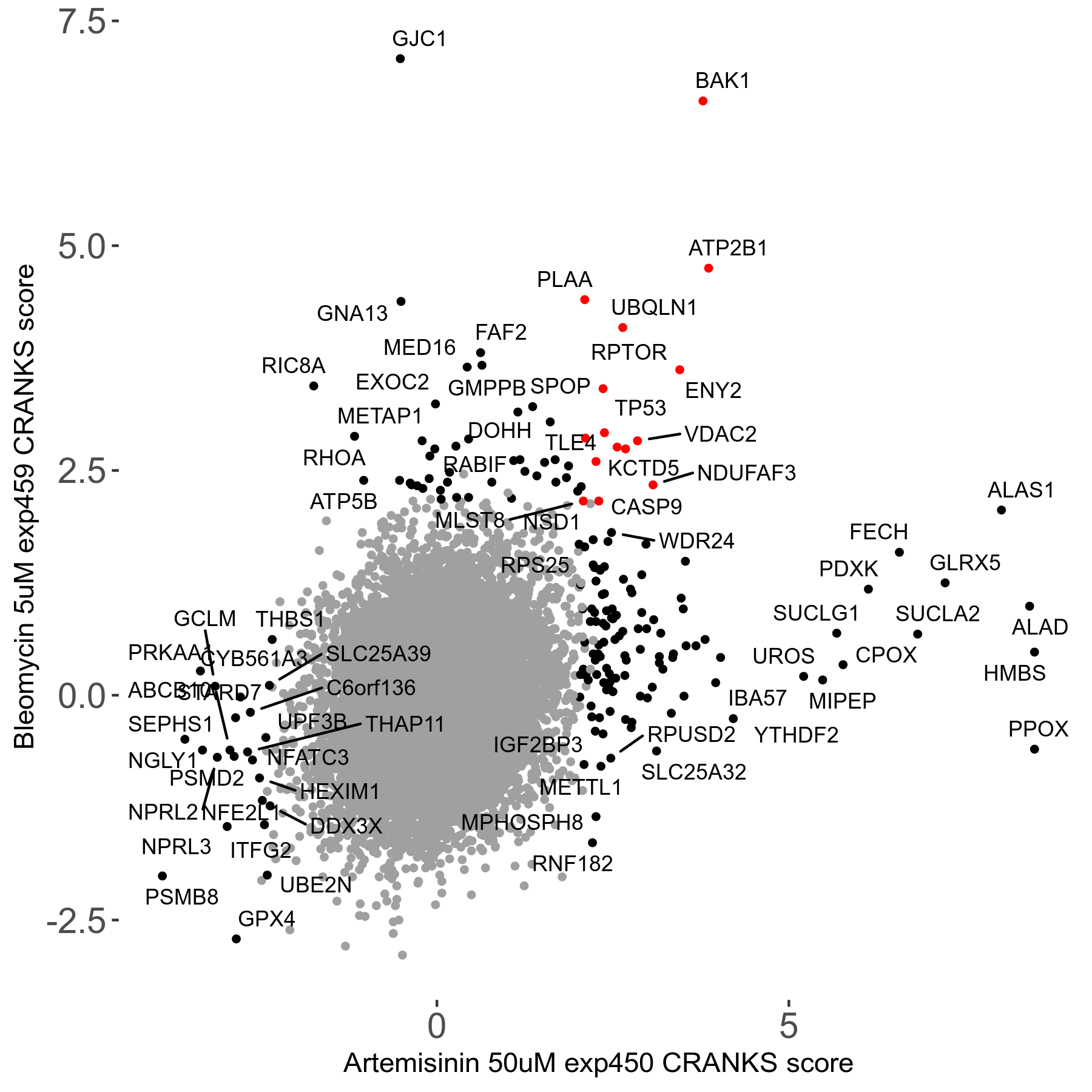
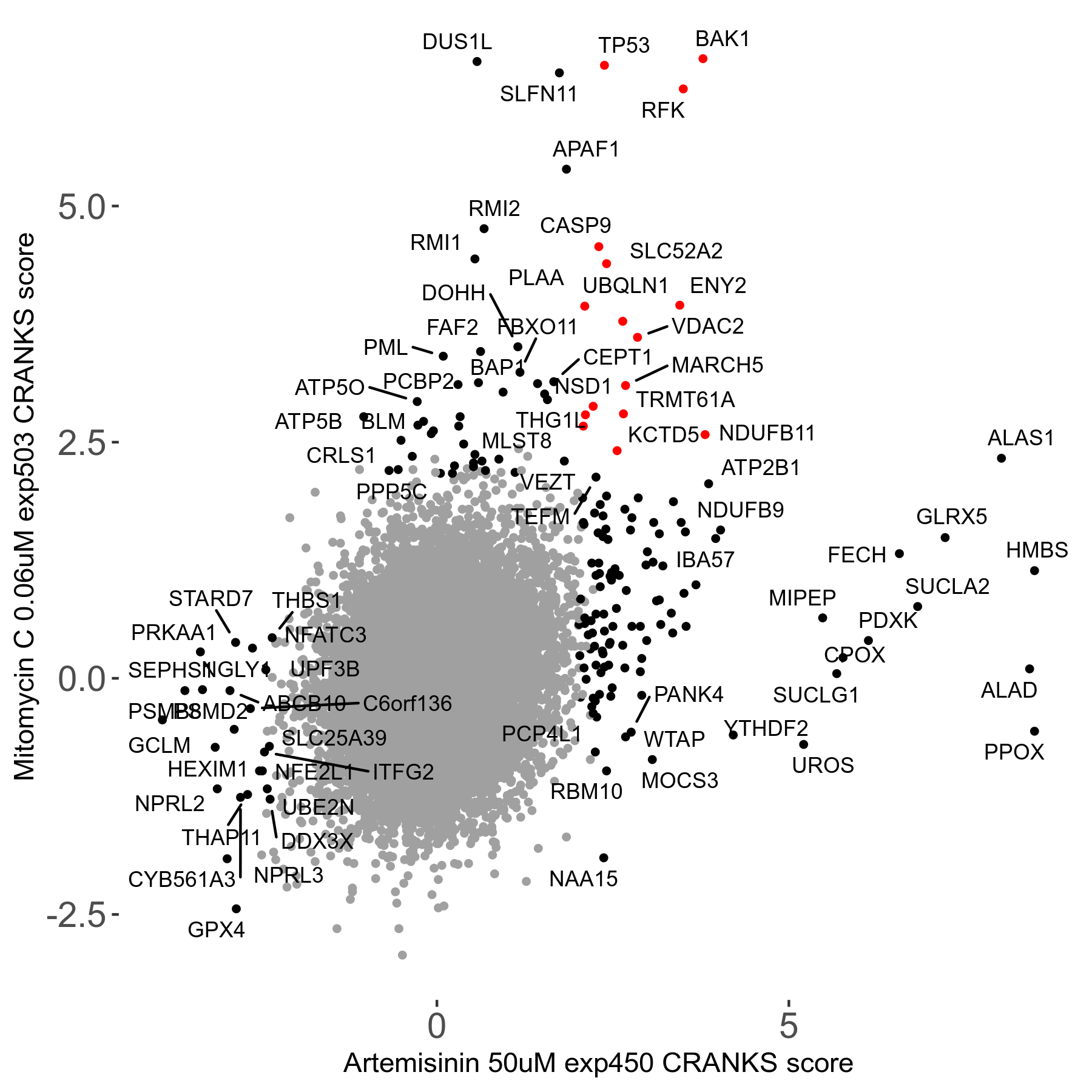
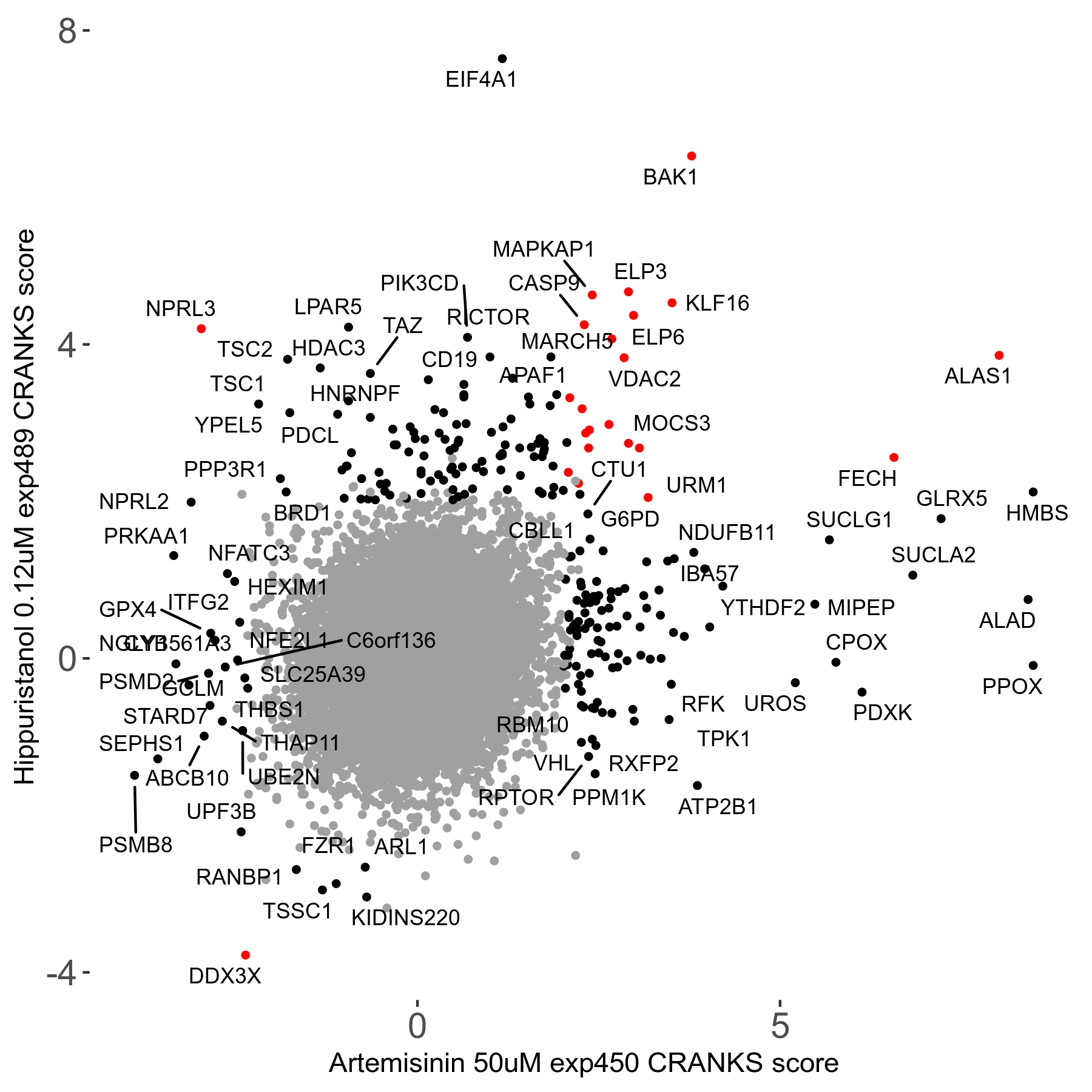
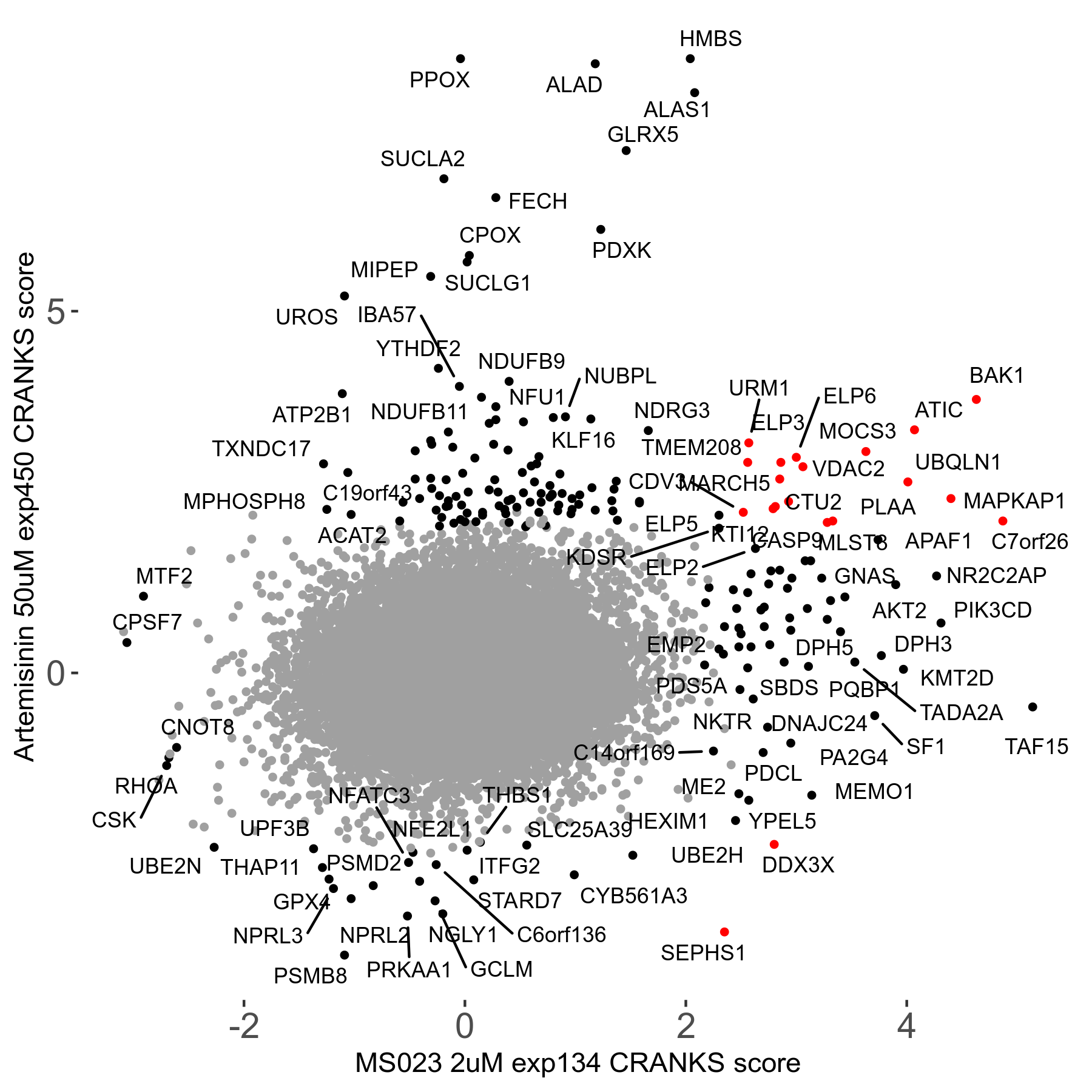
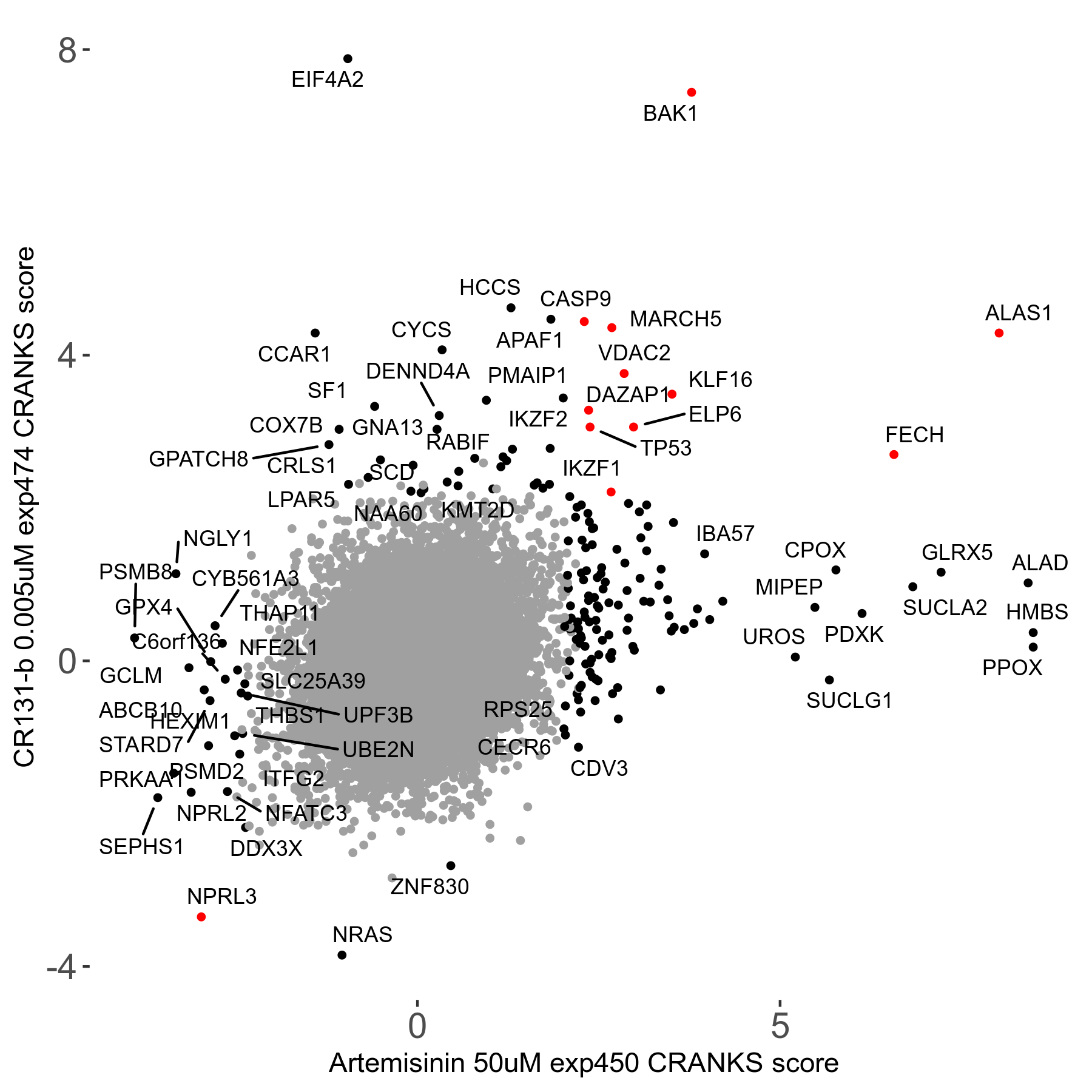
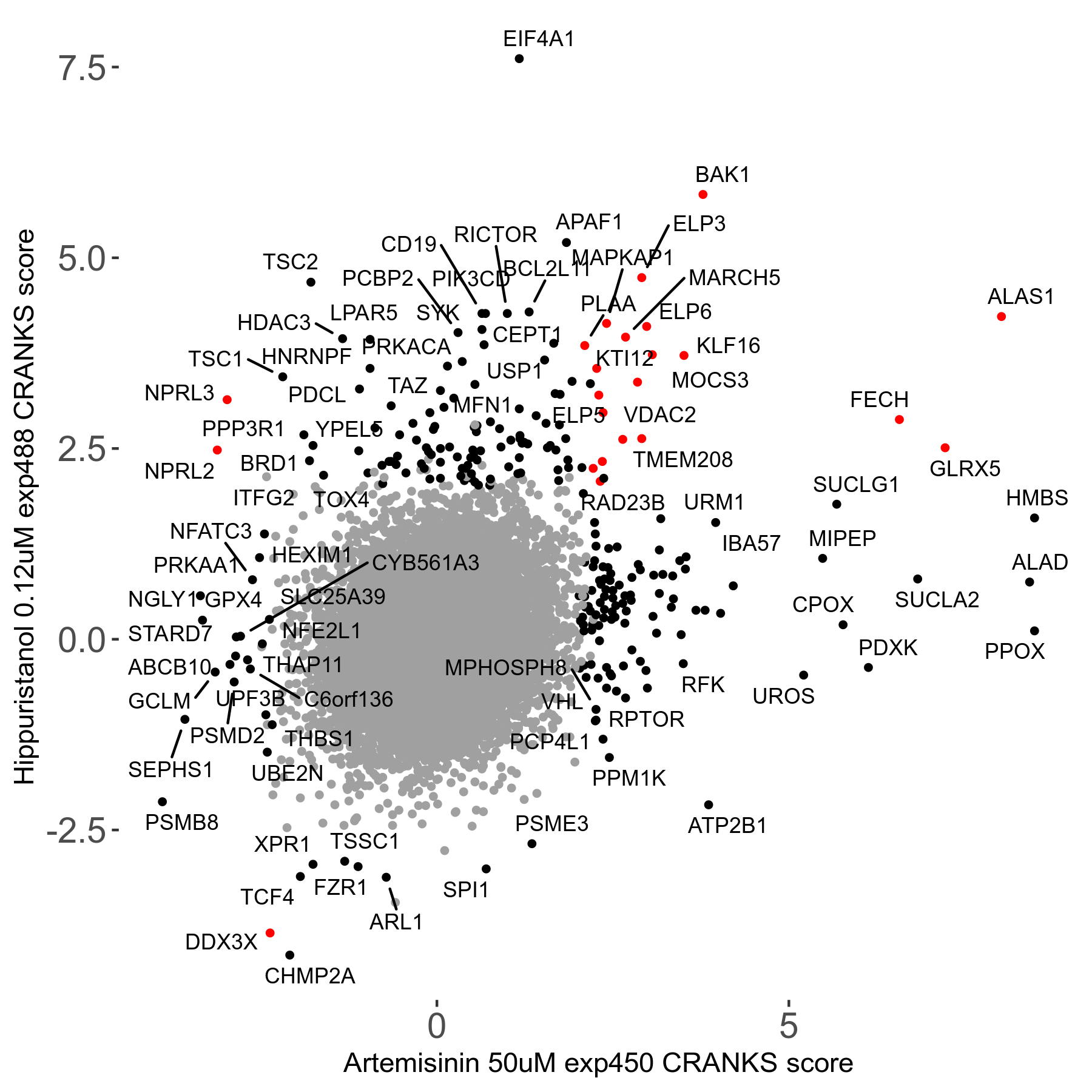
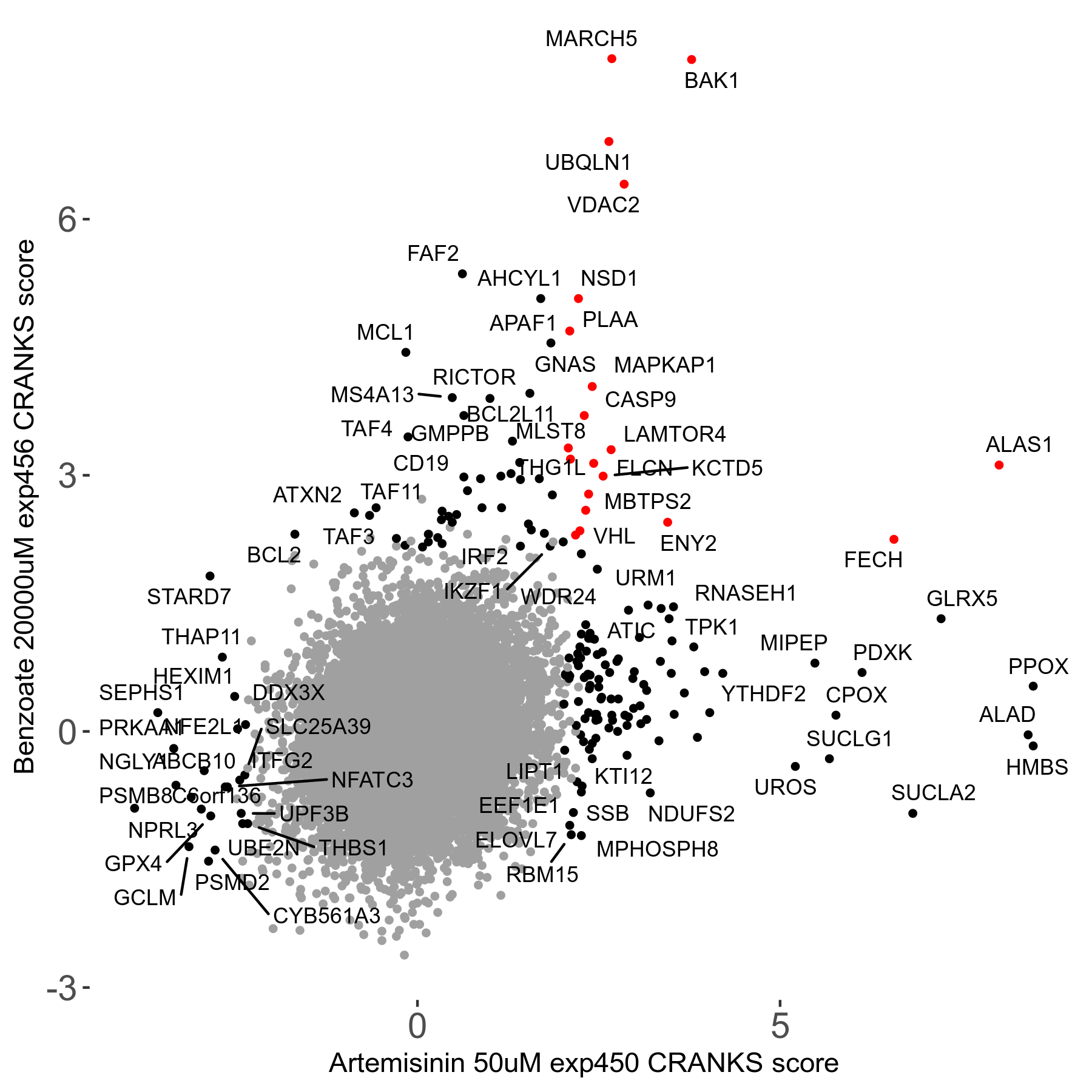
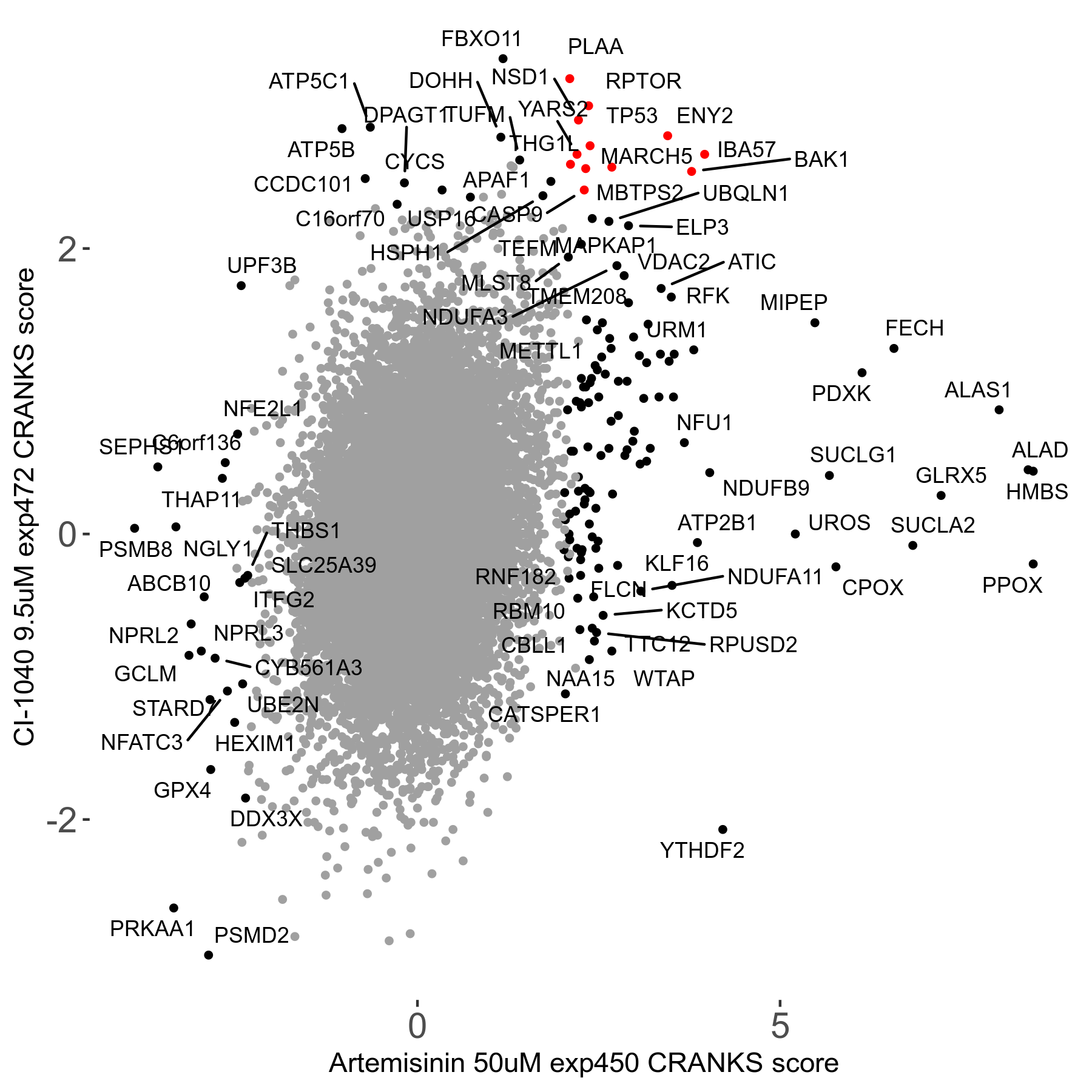
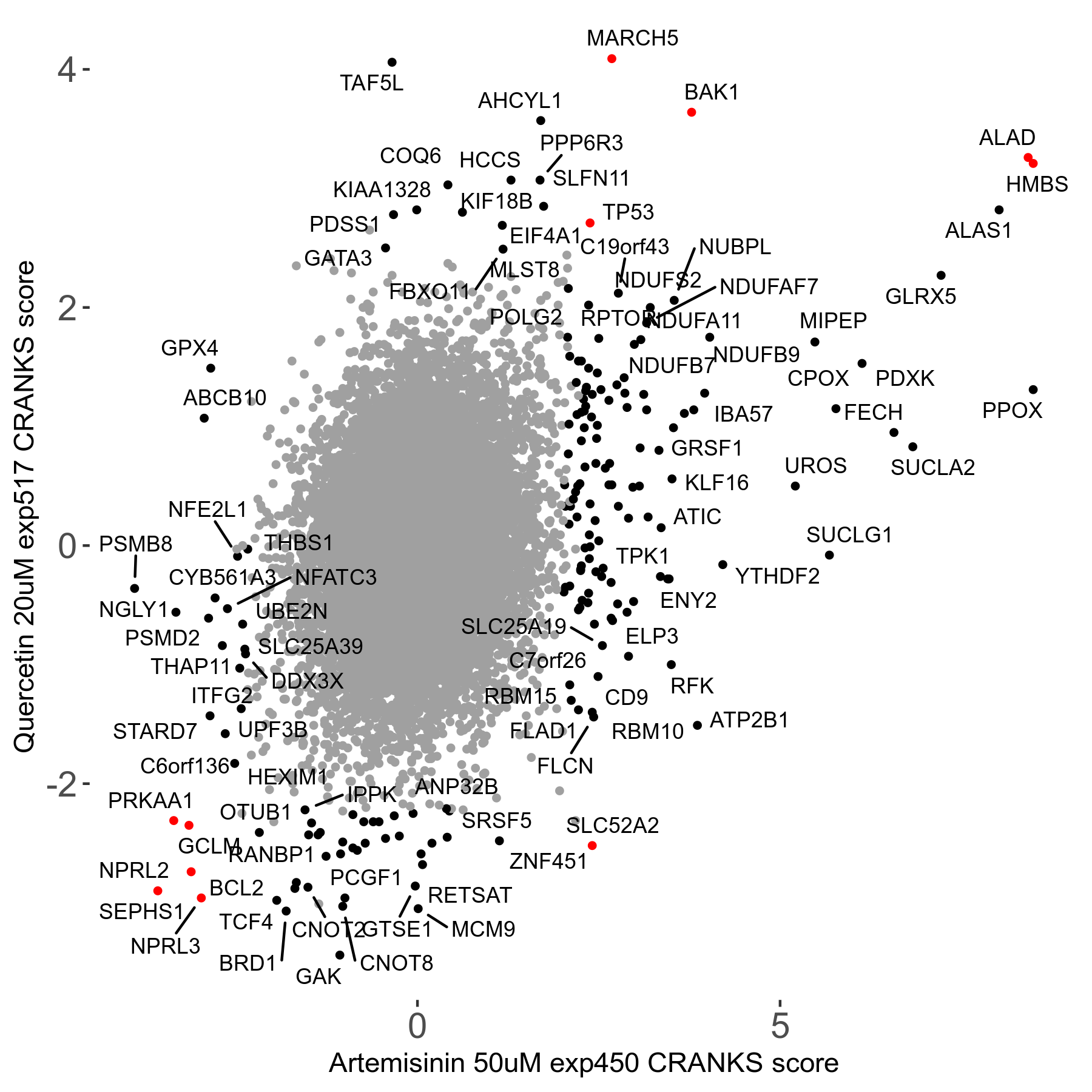
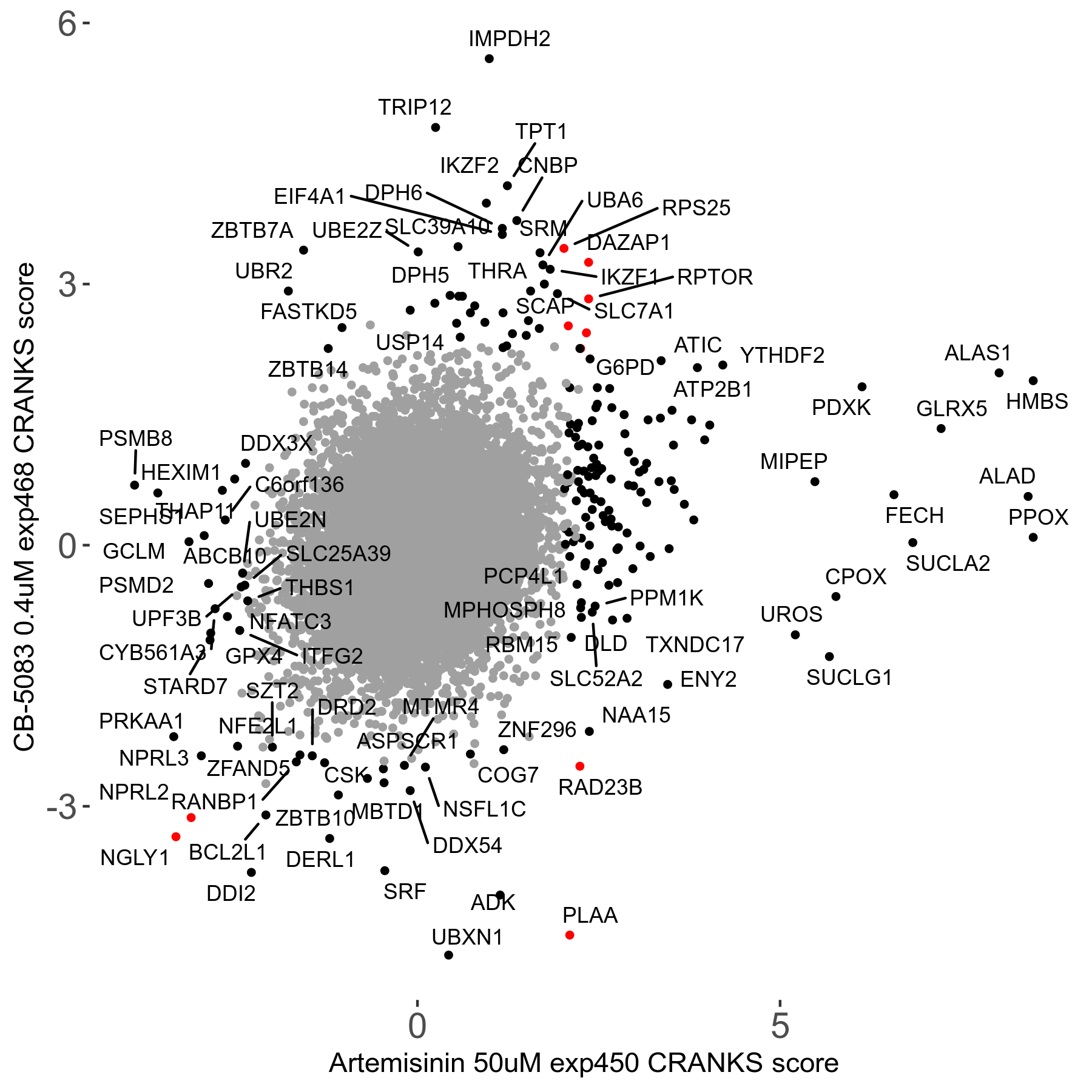
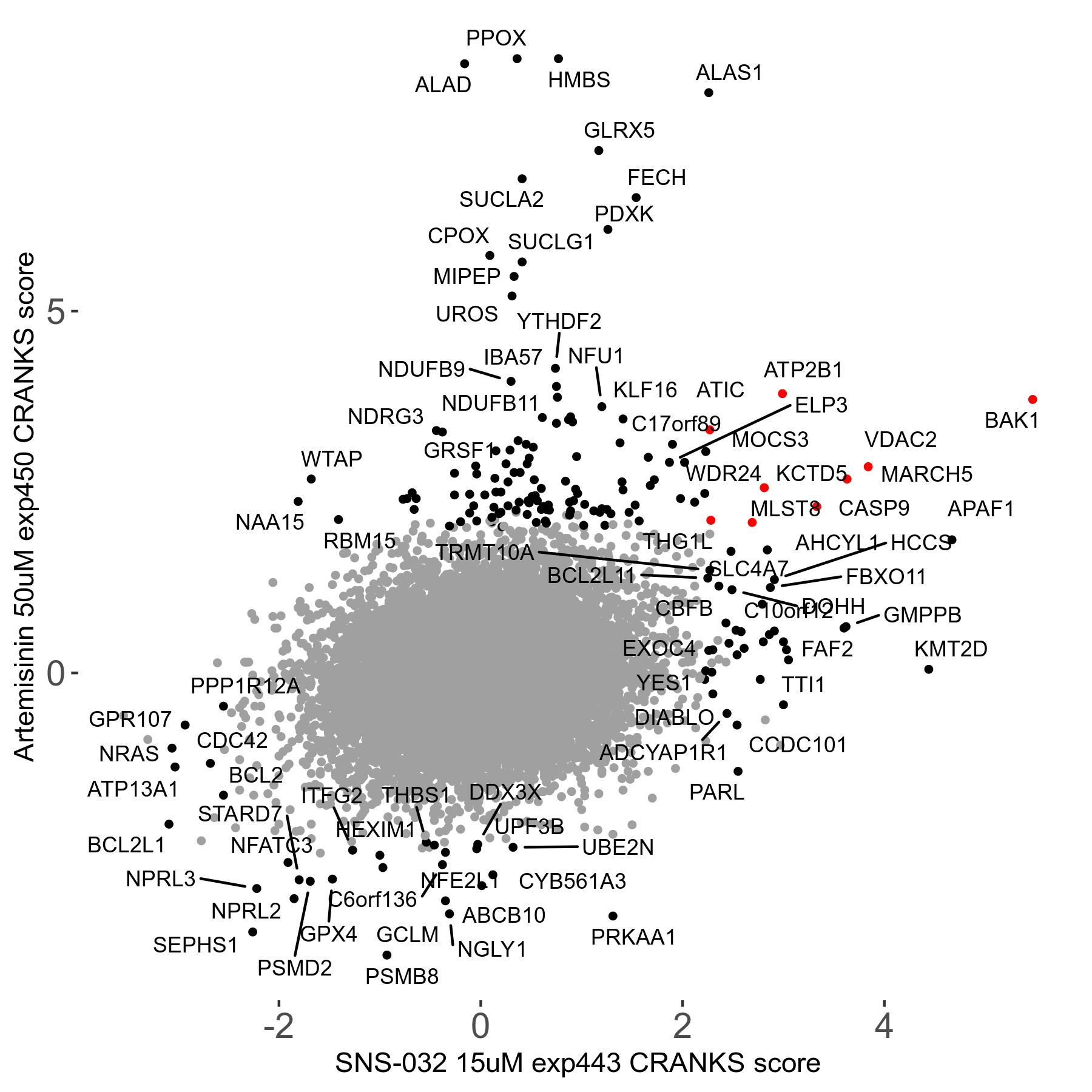
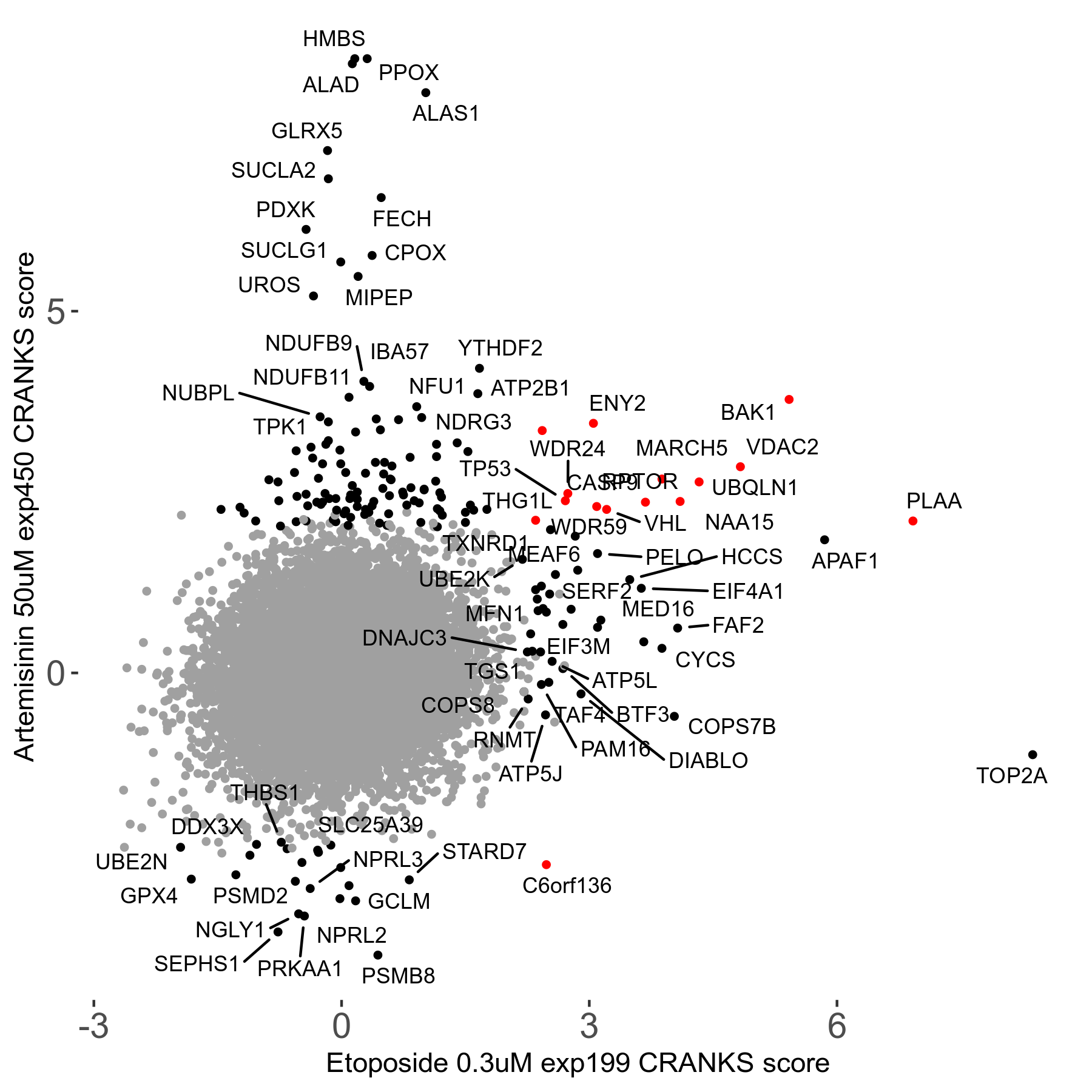
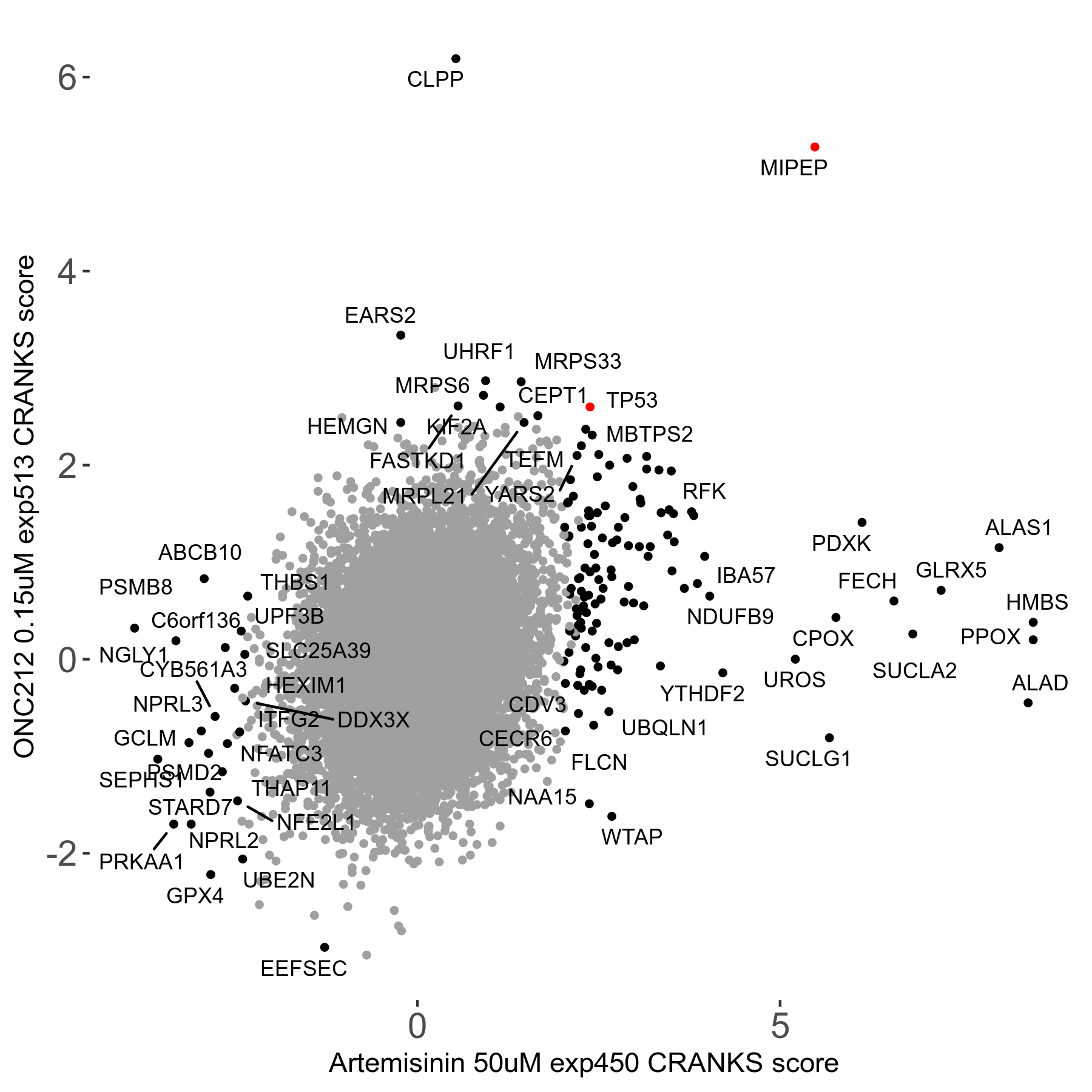
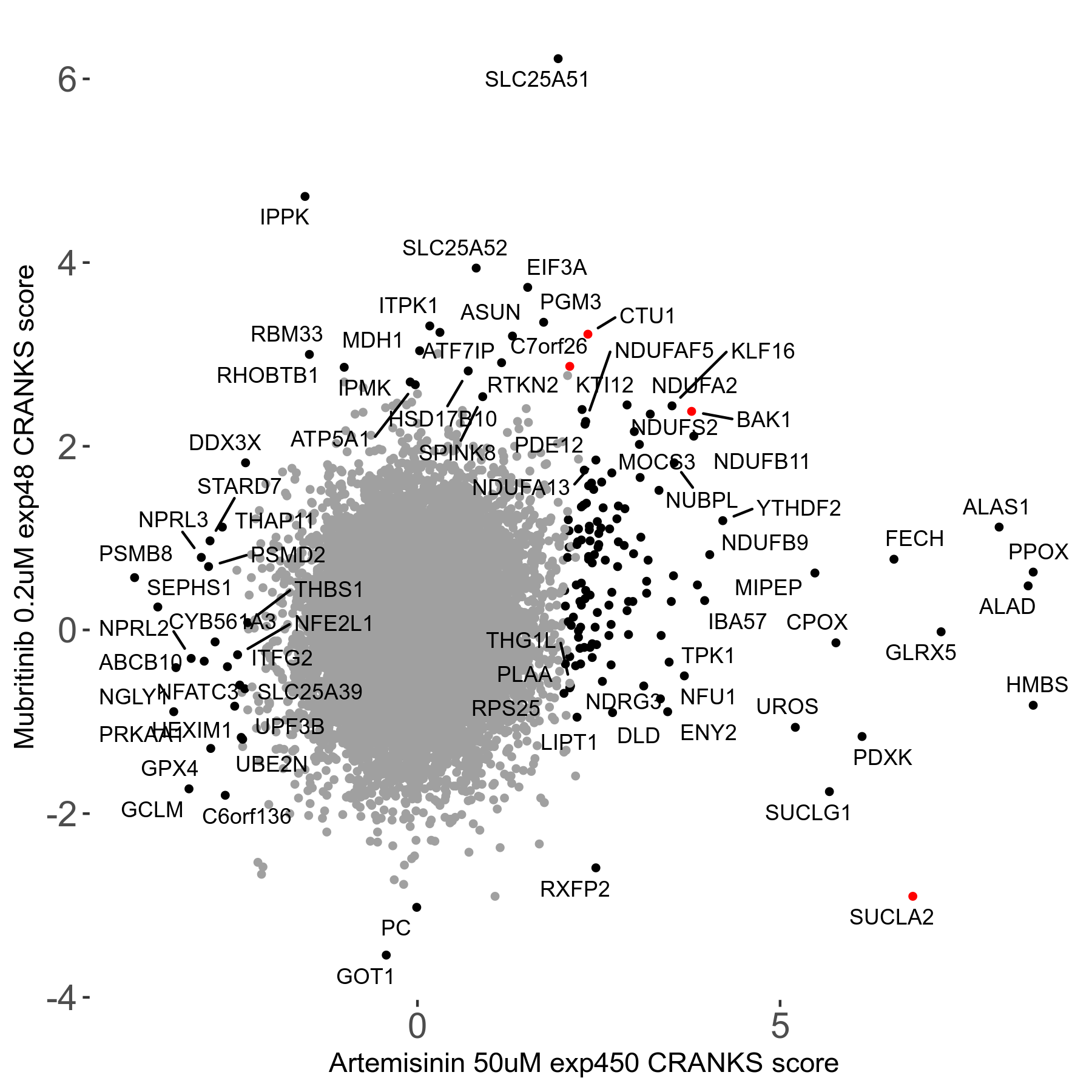
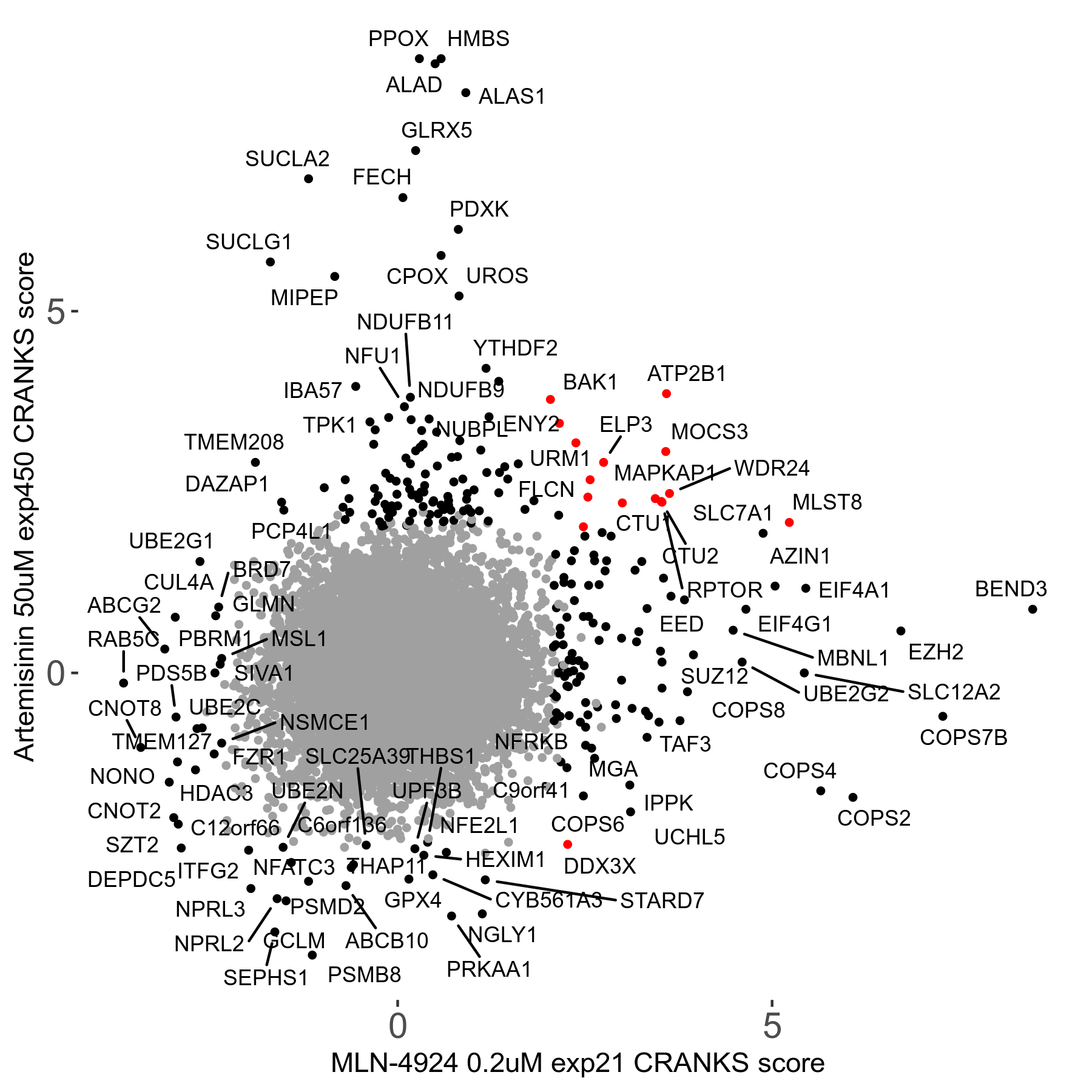
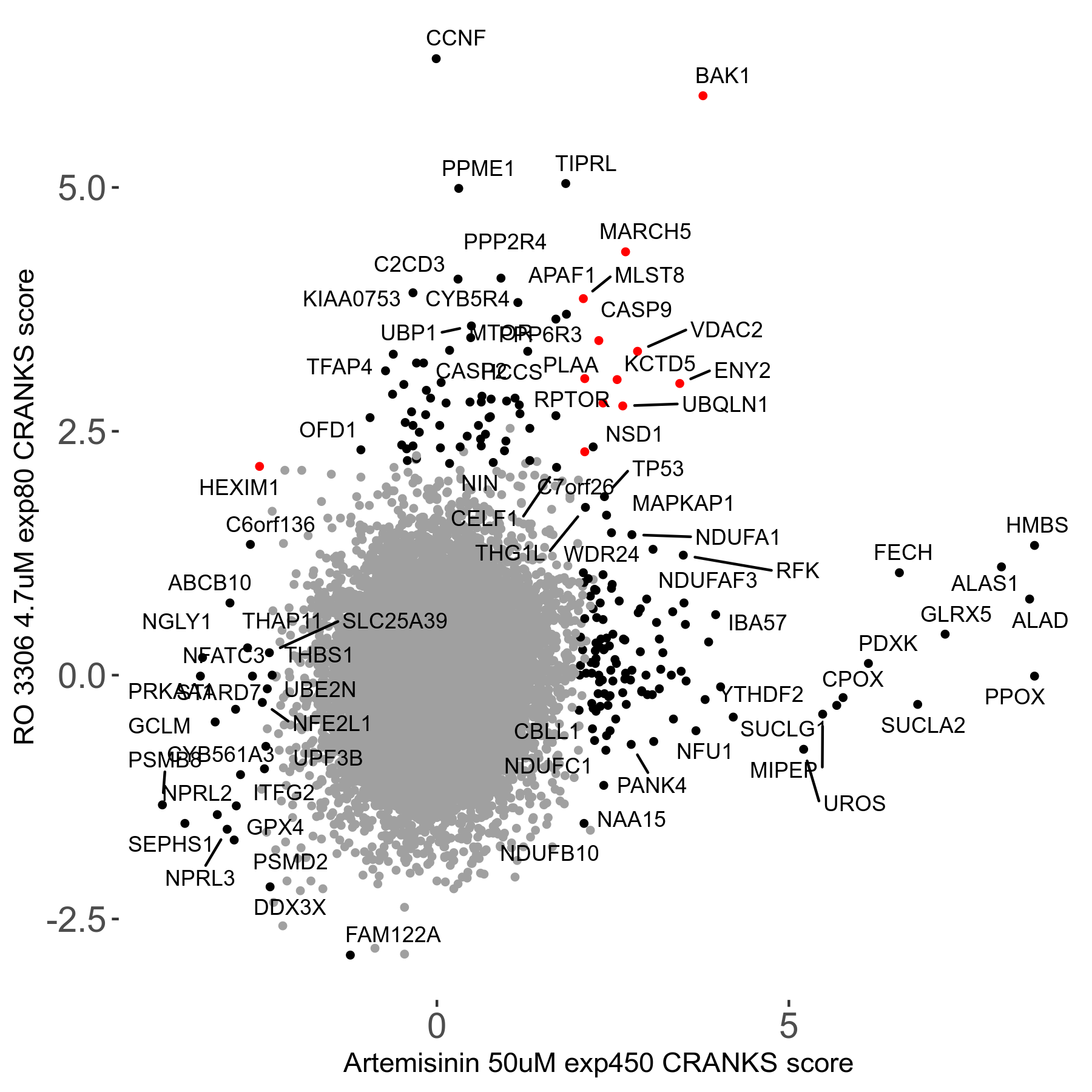
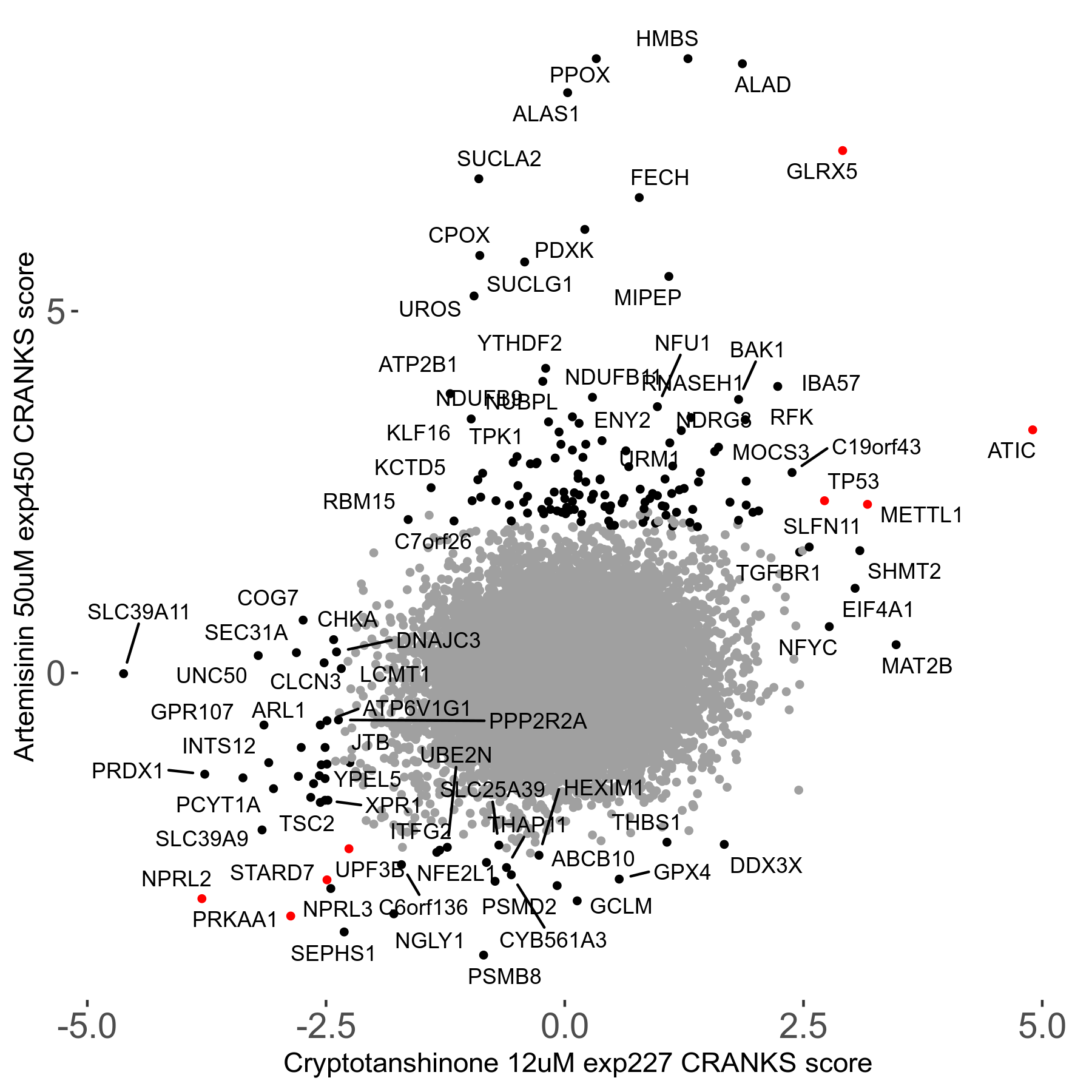
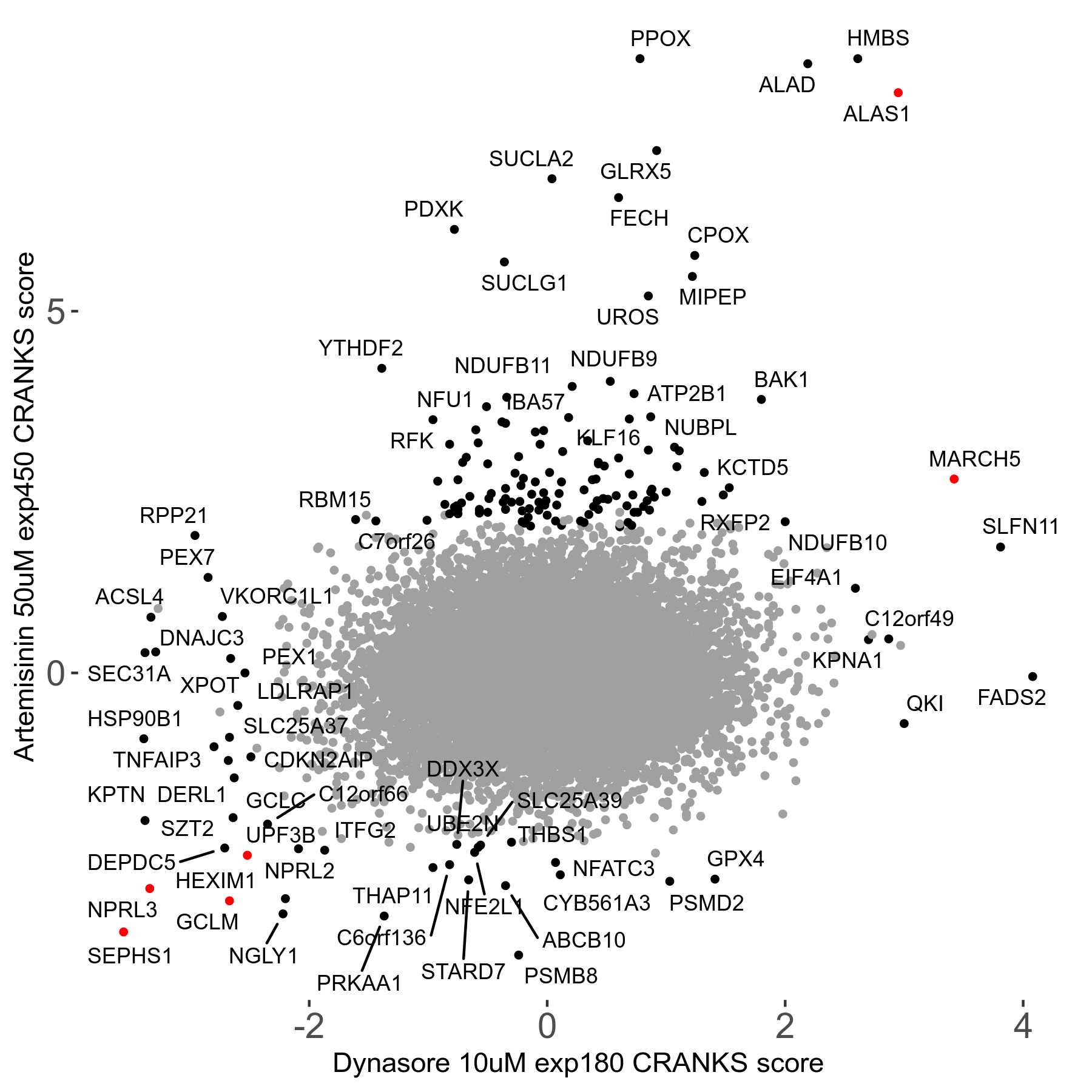
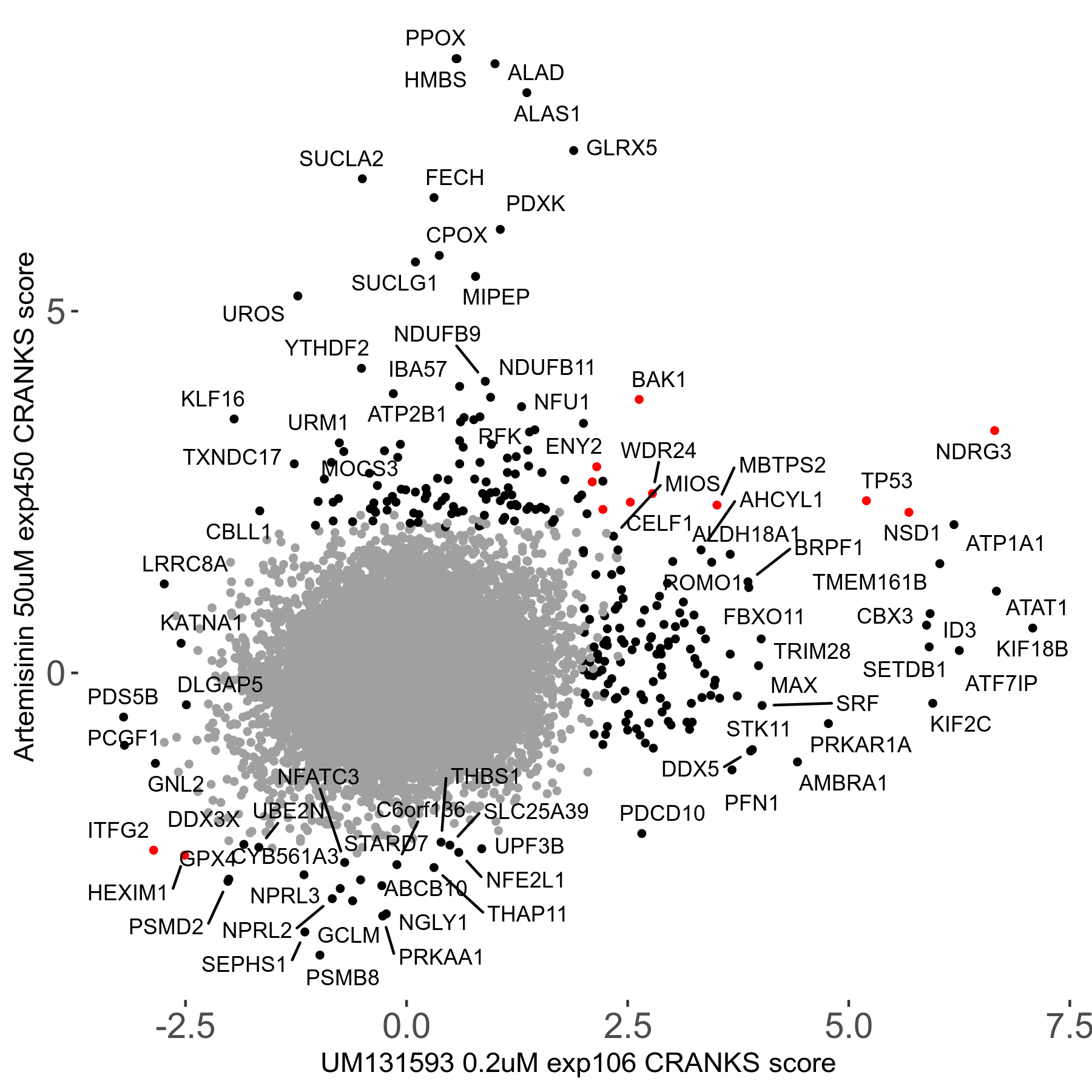
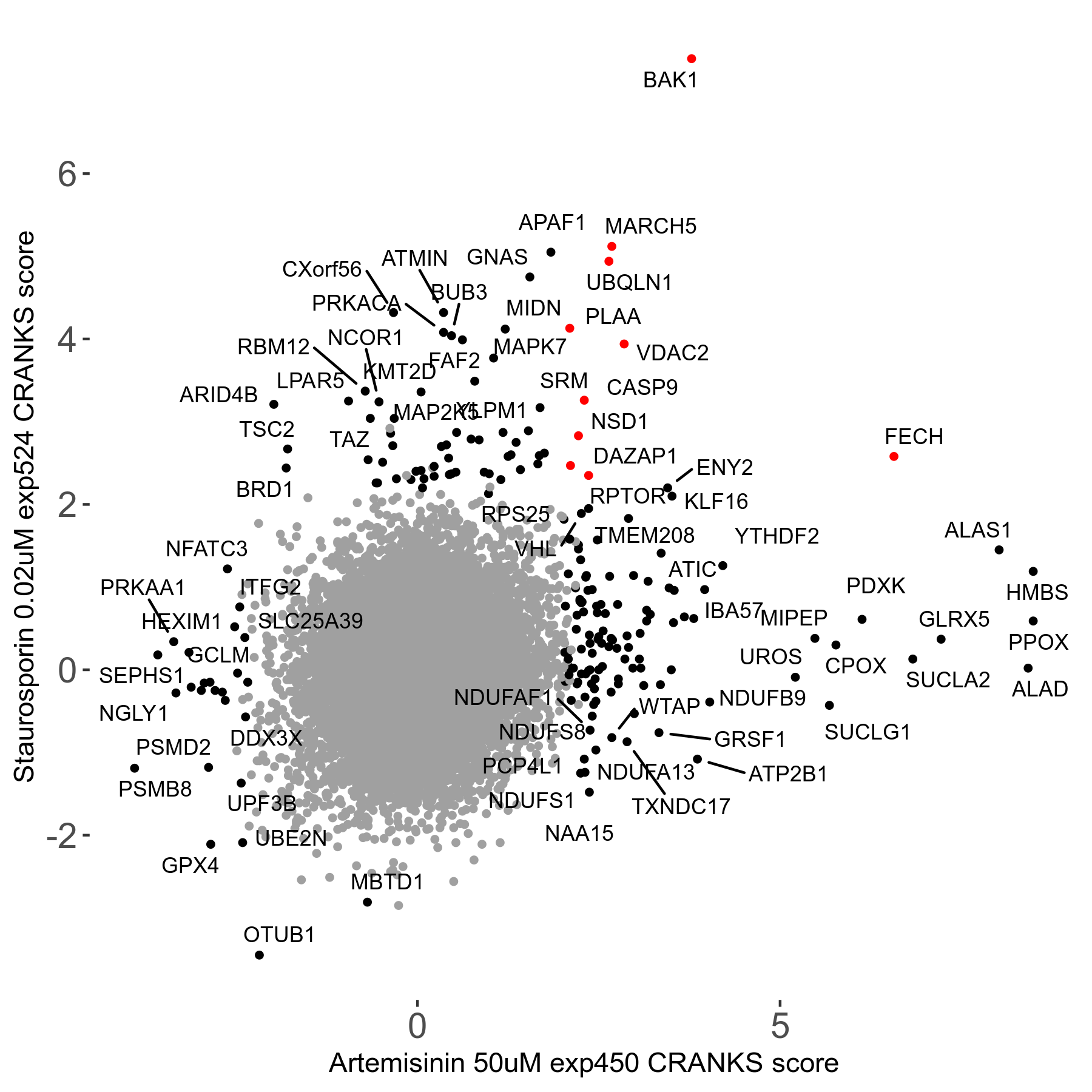
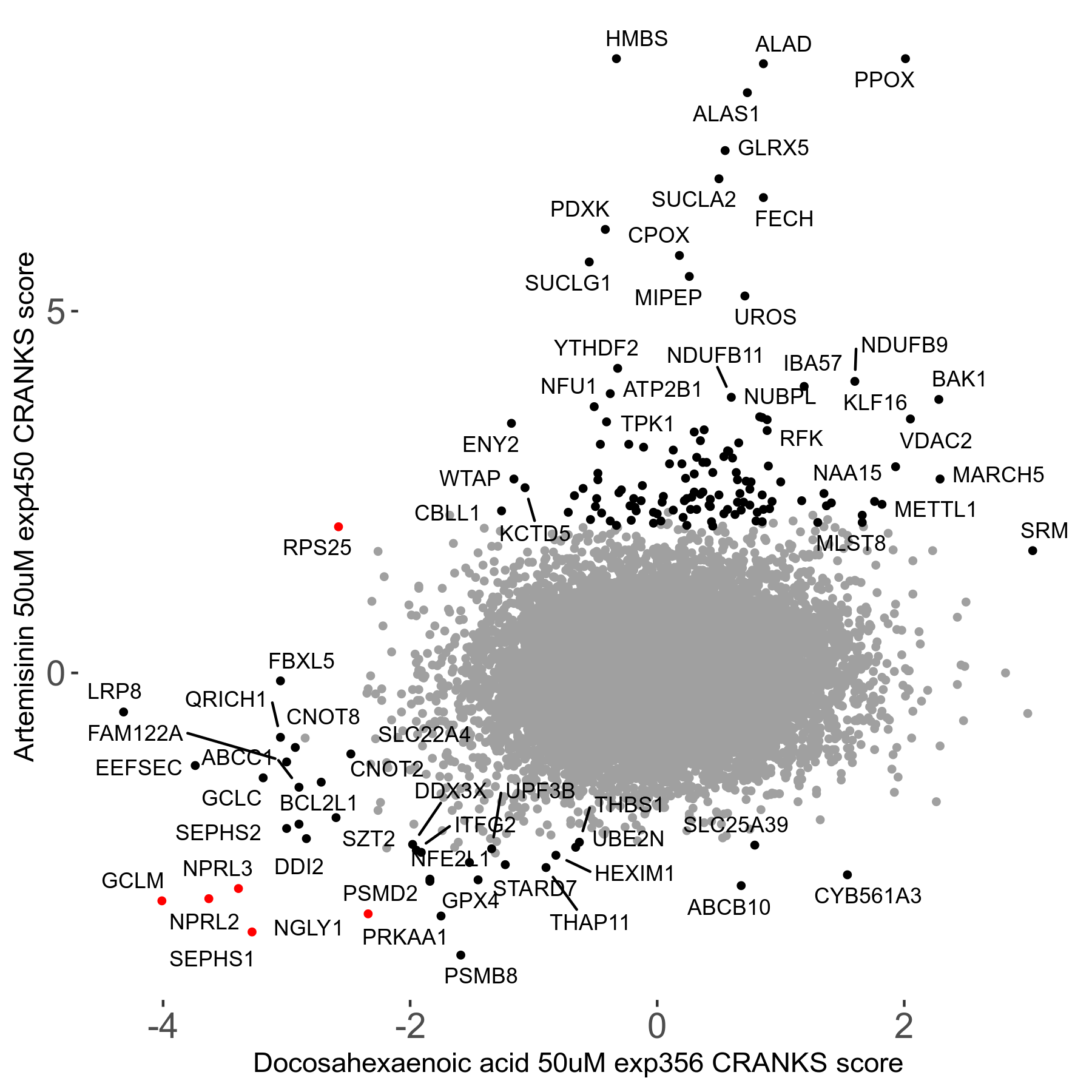
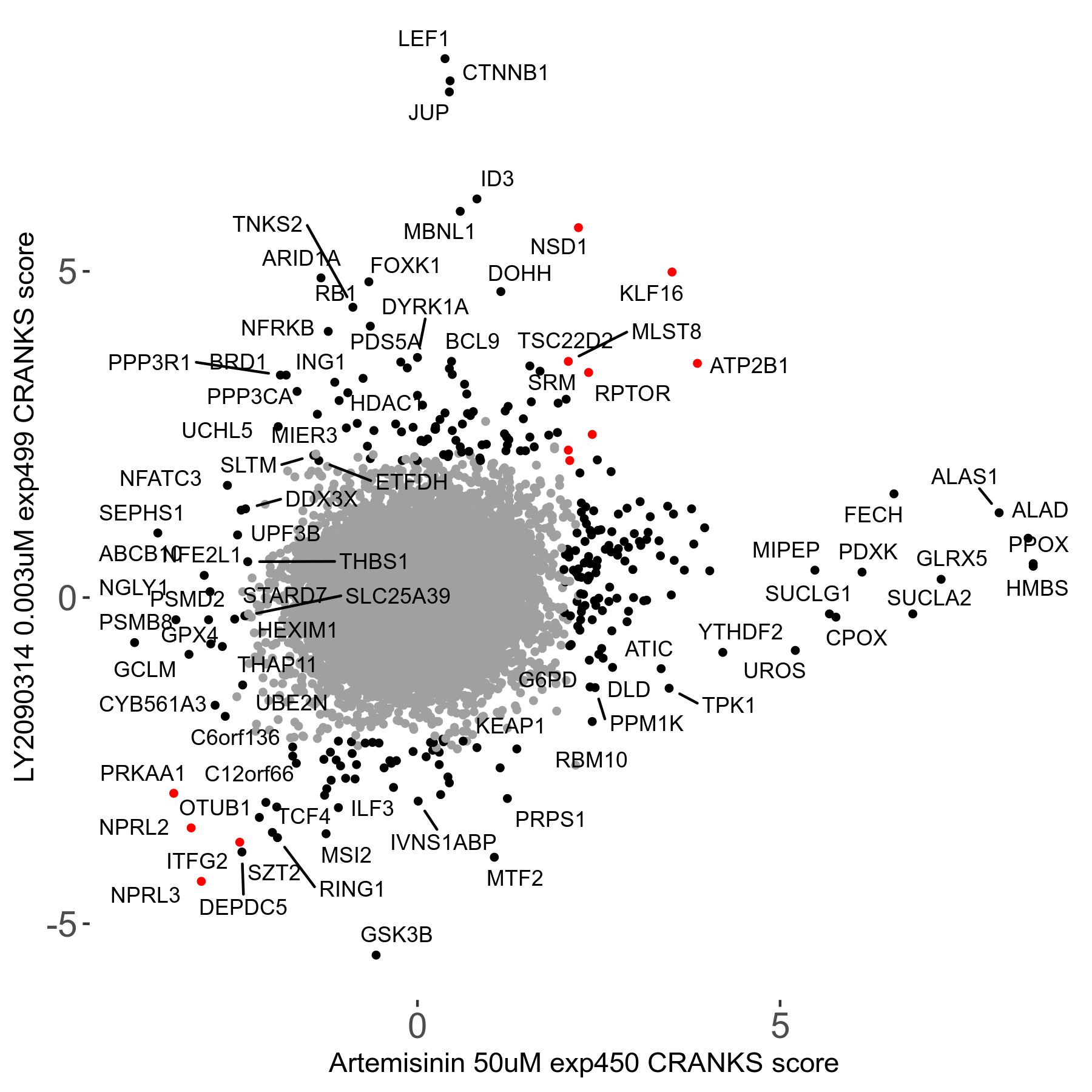
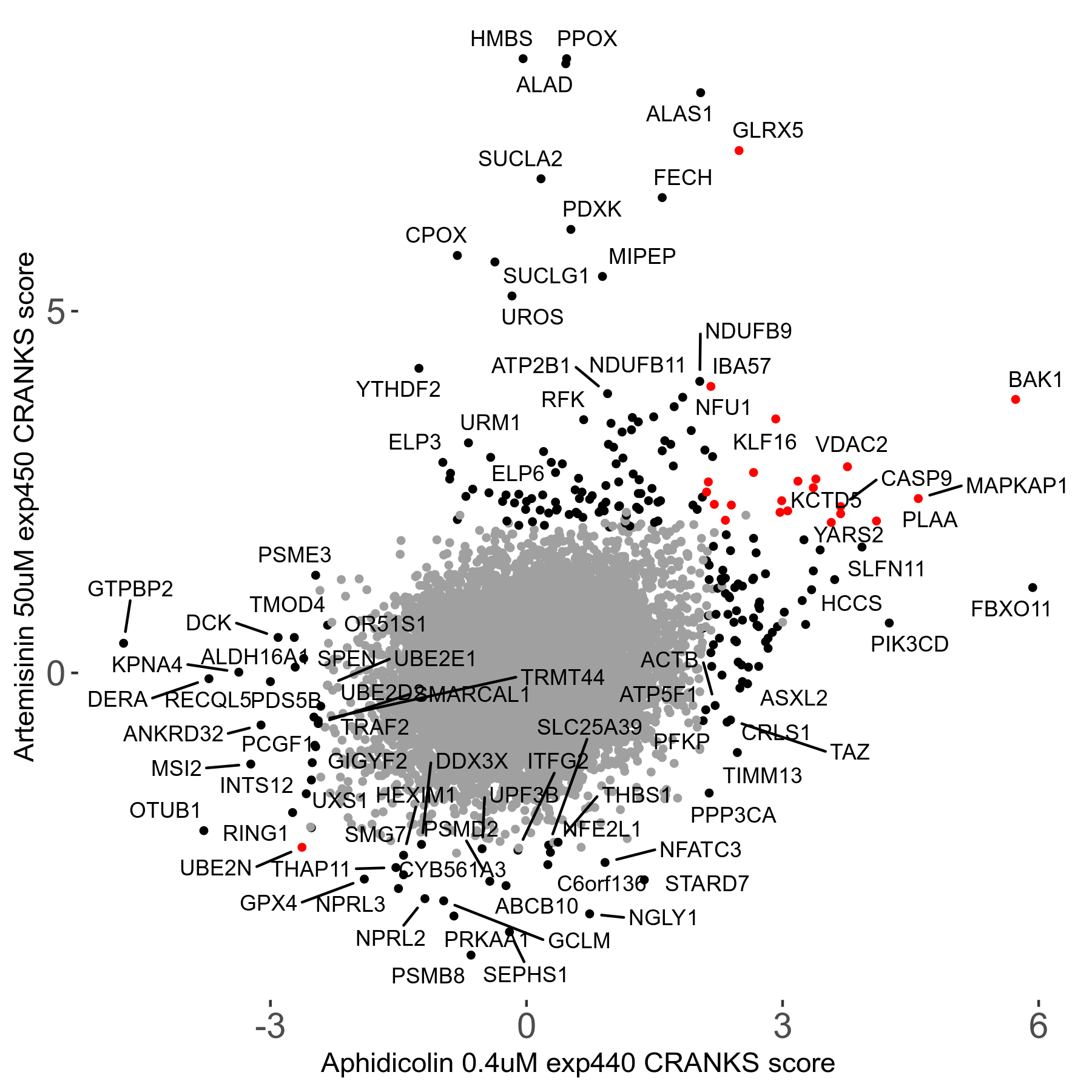
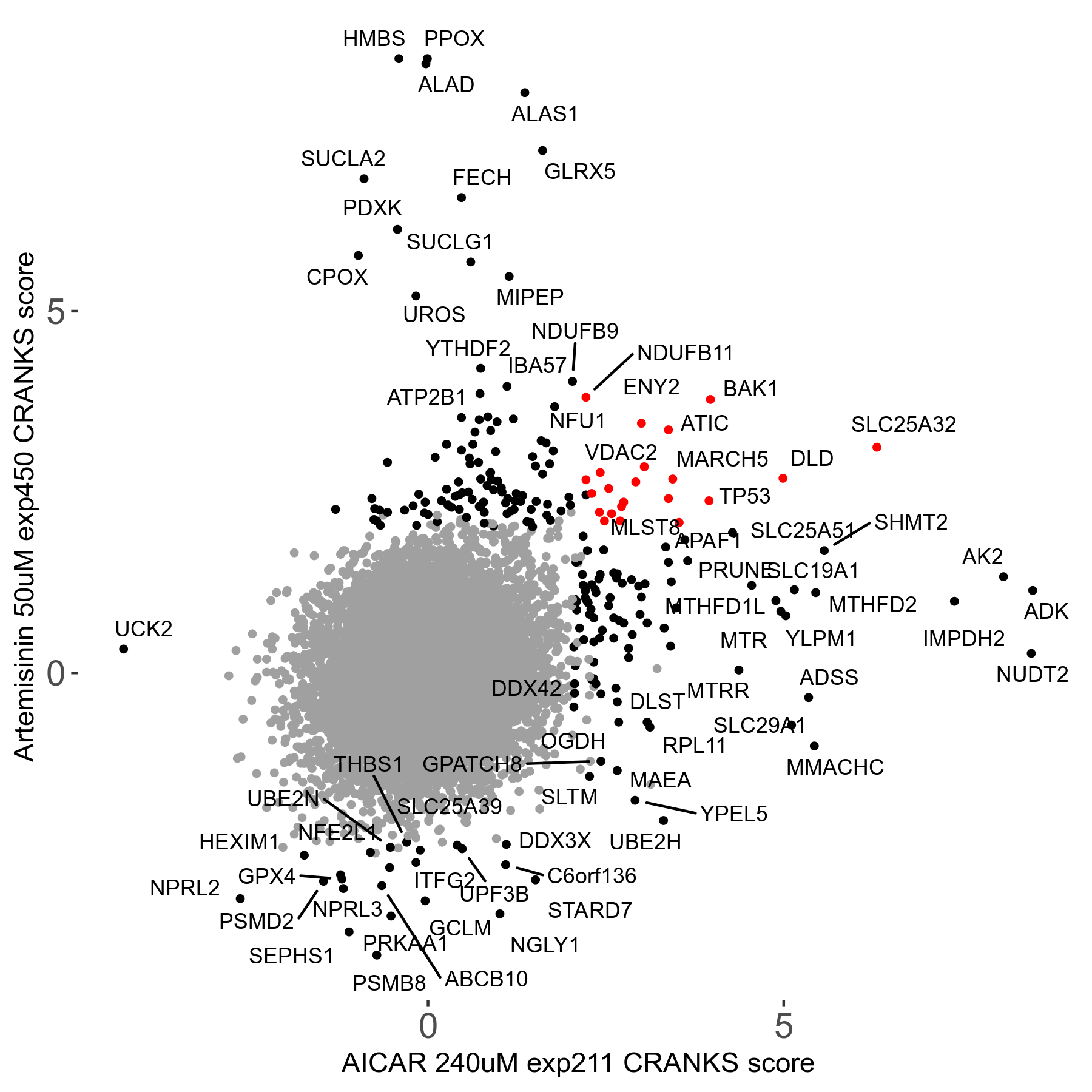
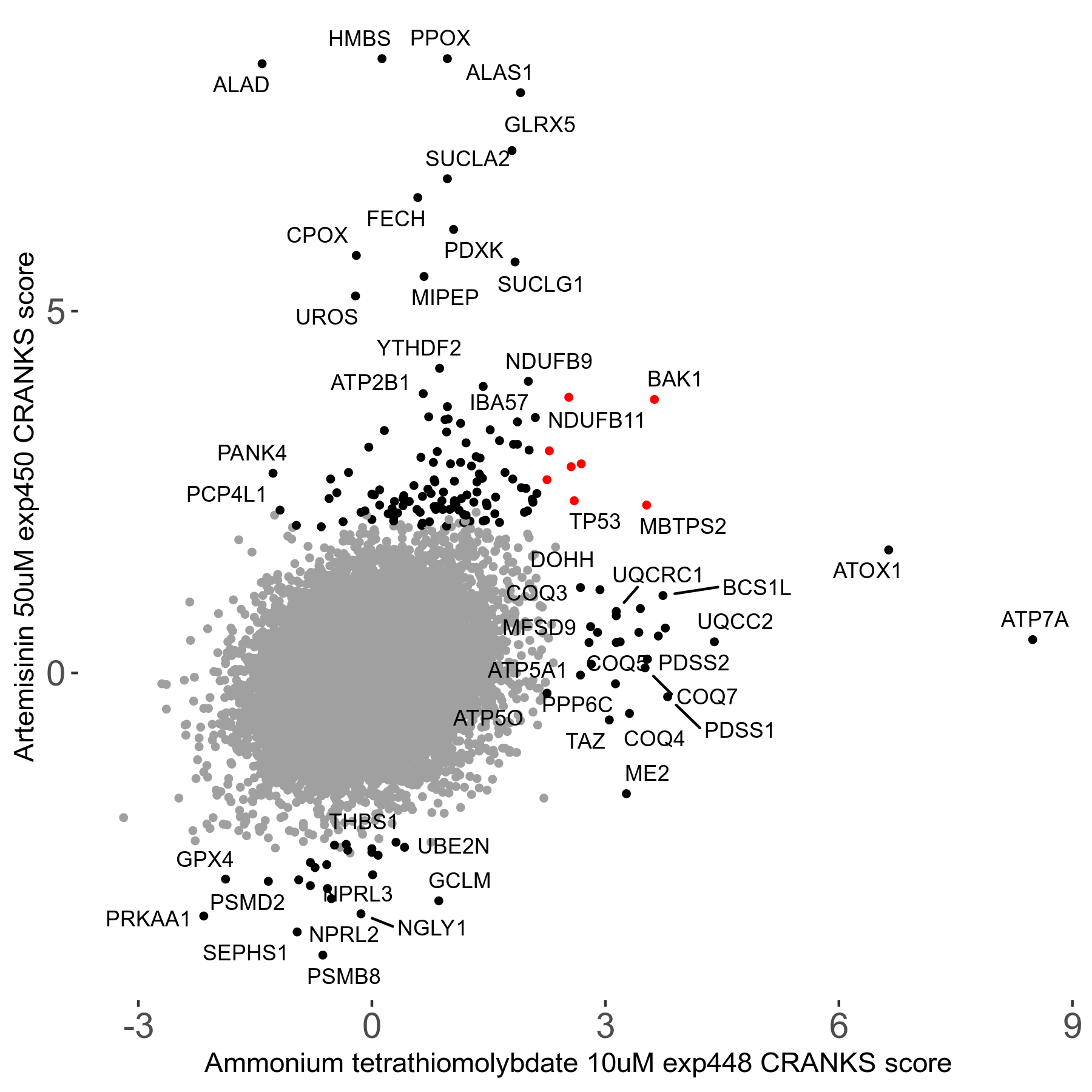
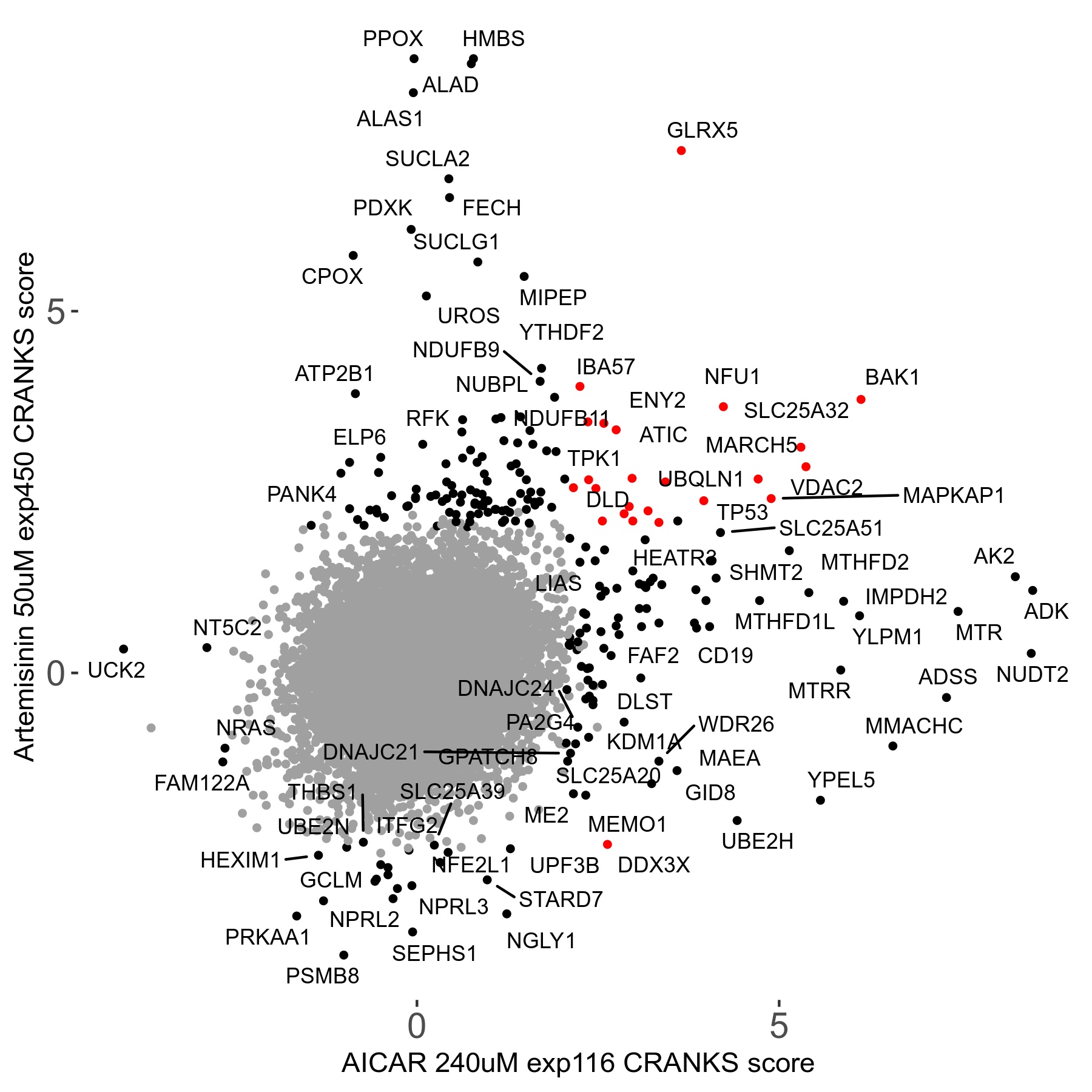
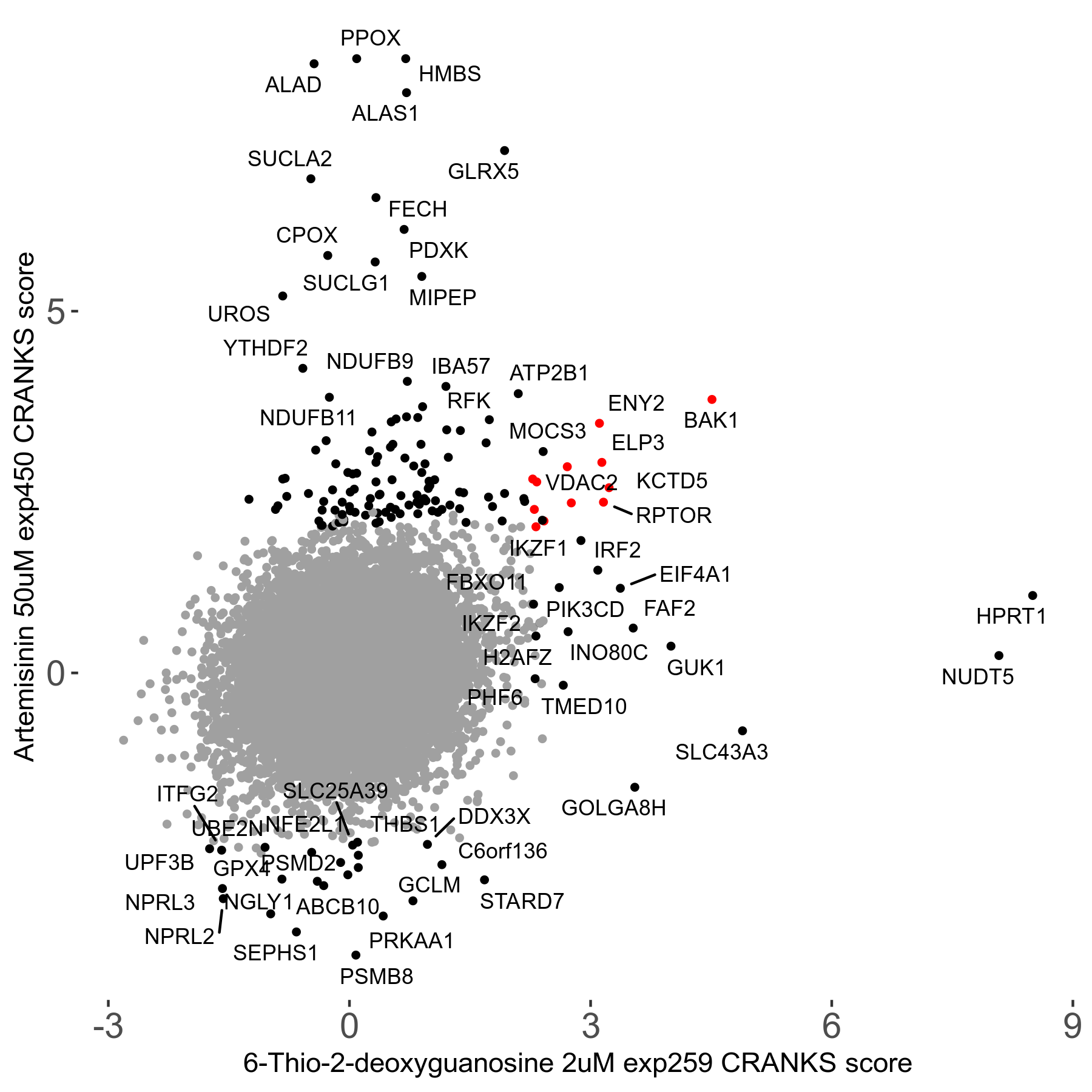
Screen Results
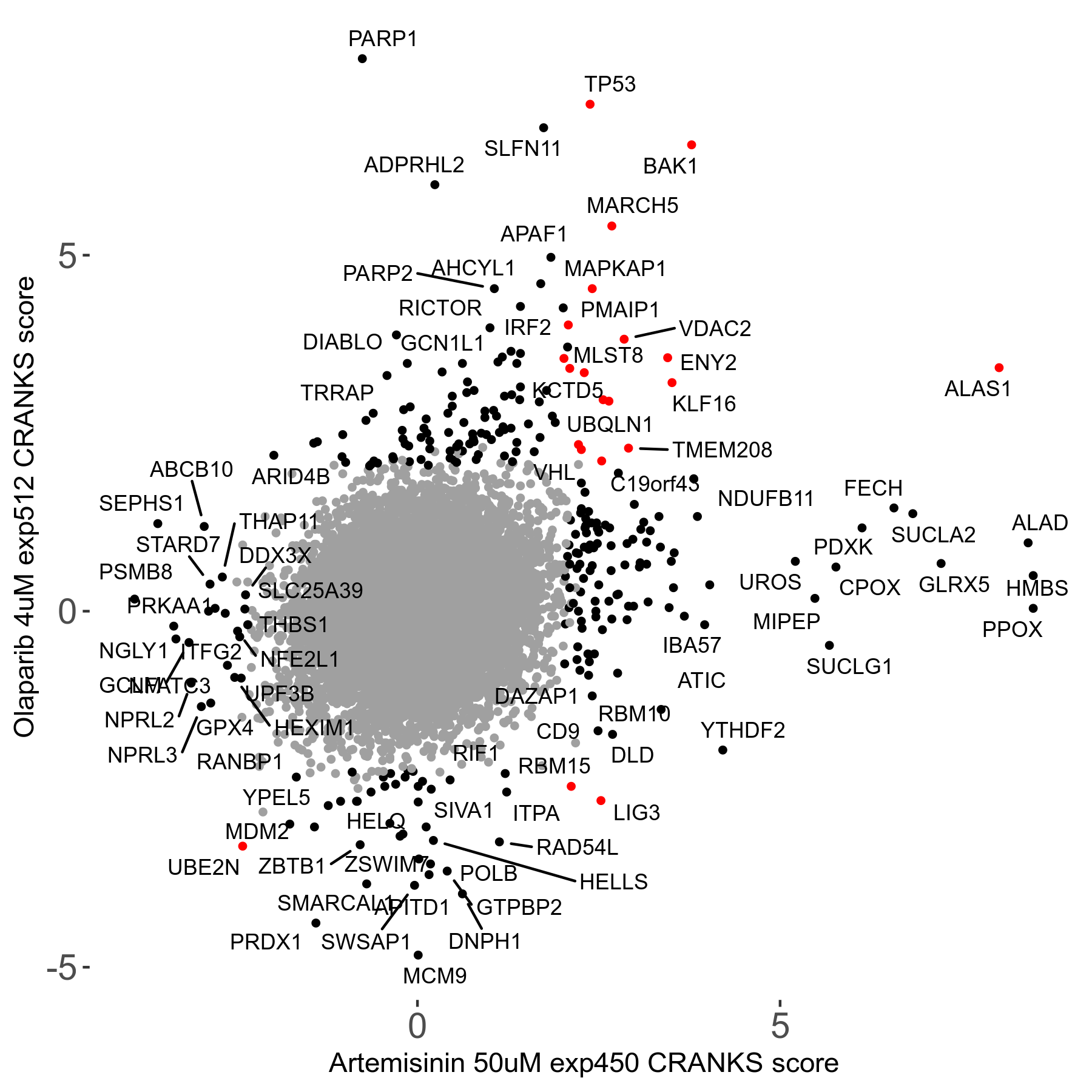
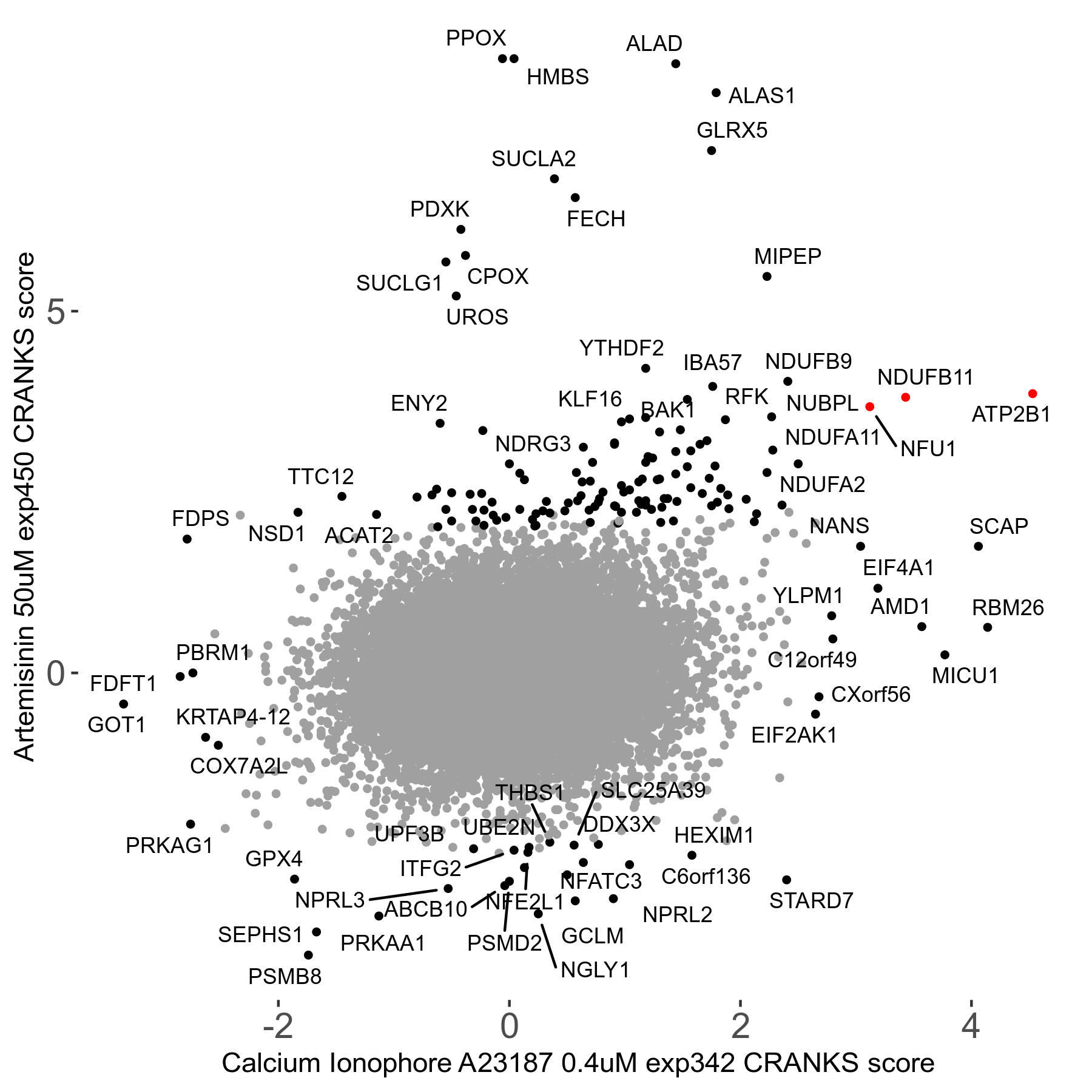
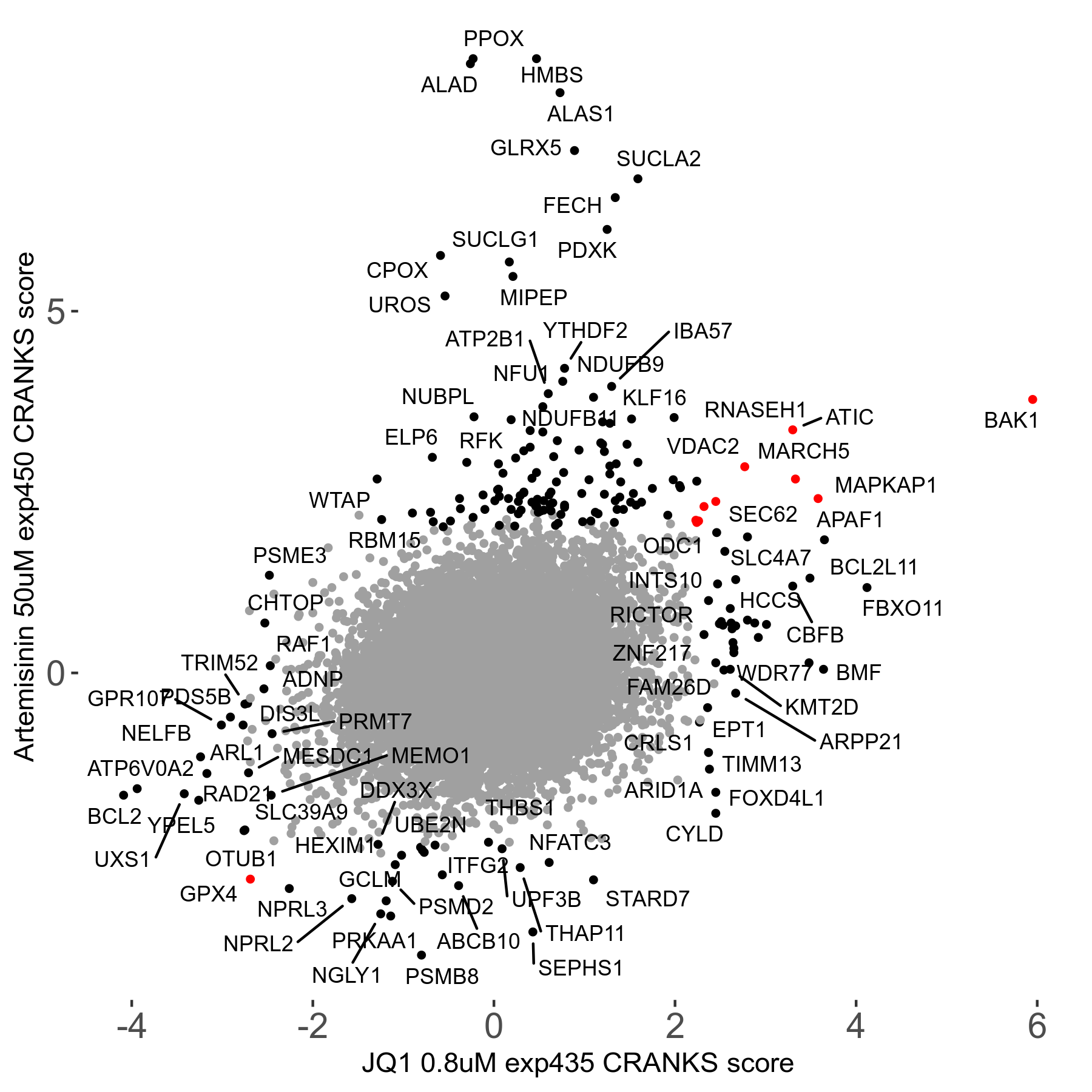
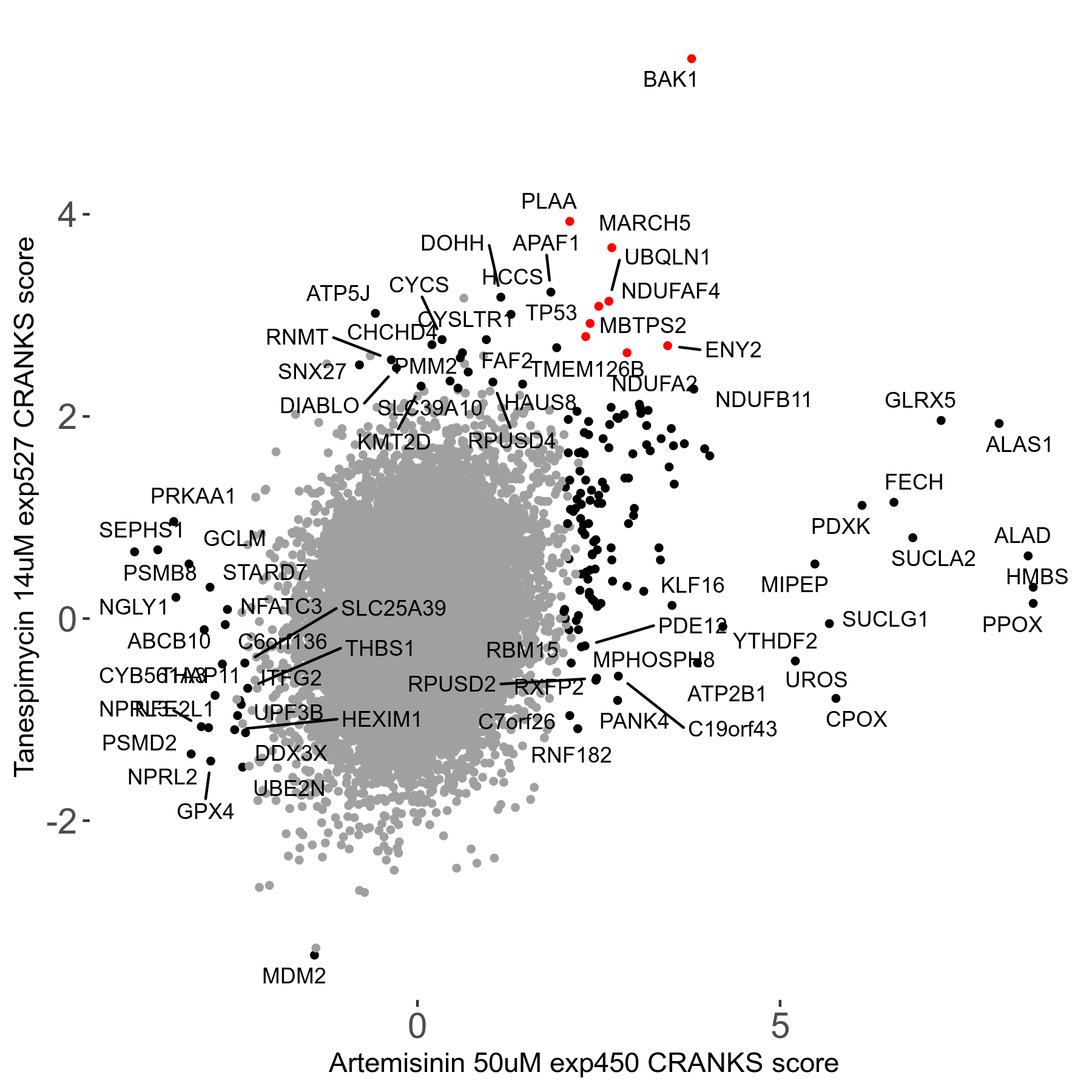
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 23/126 | Scores |