Adefovir 20μM R07 exp332
Mechanism of Action
Nucleoside reverse transcriptase inhibitor (NRTI), active against HBV and HSV
- Class / Subclass 1: Infectious Disease / Antiviral
- Class / Subclass 2: DNA Damage, Repair and Replication / Reverse Transcriptase Inhibitor
- Class / Subclass 3: Metabolism / Antimetabolite
Technical Notes
Compound References
- PubChem Name: Adefovir
- Synonyms: GS-0393; PMEA
- CAS #: 106941-25-7
- PubChem CID: 60172
- IUPAC: 2-(6-aminopurin-9-yl)ethoxymethylphosphonic acid
- INCHI Name: InChI=1S/C8H12N5O4P/c9-7-6-8(11-3-10-7)13(4-12-6)1-2-17-5-18(14,15)16/h3-4H,1-2,5H2,(H2,9,10,11)(H2,14,15,16)
- INCHI Key: SUPKOOSCJHTBAH-UHFFFAOYSA-N
- Molecular Weight: 273.19
- Canonical SMILES: C1=NC(=C2C(=N1)N(C=N2)CCOCP(=O)(O)O)N
- Isomeric SMILES: N/A
- Molecular Formula: C8H12N5O4P
Compound Supplier
- Supplier Name: Ark Pharm Inc.
- Catalog #: AK-75903
- Lot #: WG0017455-170306001
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C8H12N5O4P 274.06997; found 274.06966
Dose Response Curve
- Platform ID: Adefovir
- Min: -8.5475; Max: 70.4919

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 0.0648 |
| IC30 | 0.1411 |
| IC40 | 0.3040 |
| IC50 | 0.7276 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 07
- Dose: 20µM
- Days of incubation: 8
- Doublings: 6.3
- Numbers of reads: 22625883
Screen Results
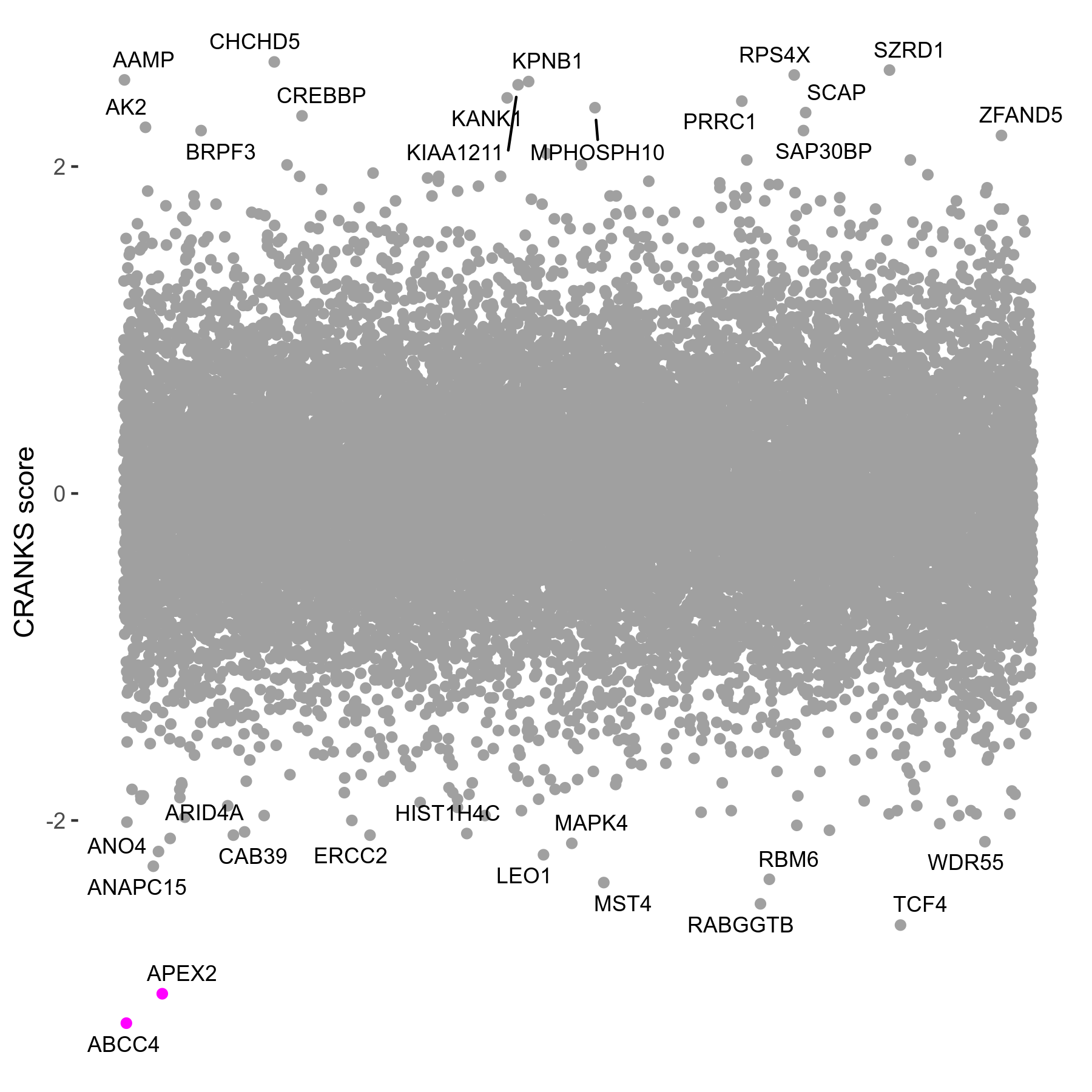
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 2/0 | Scores |