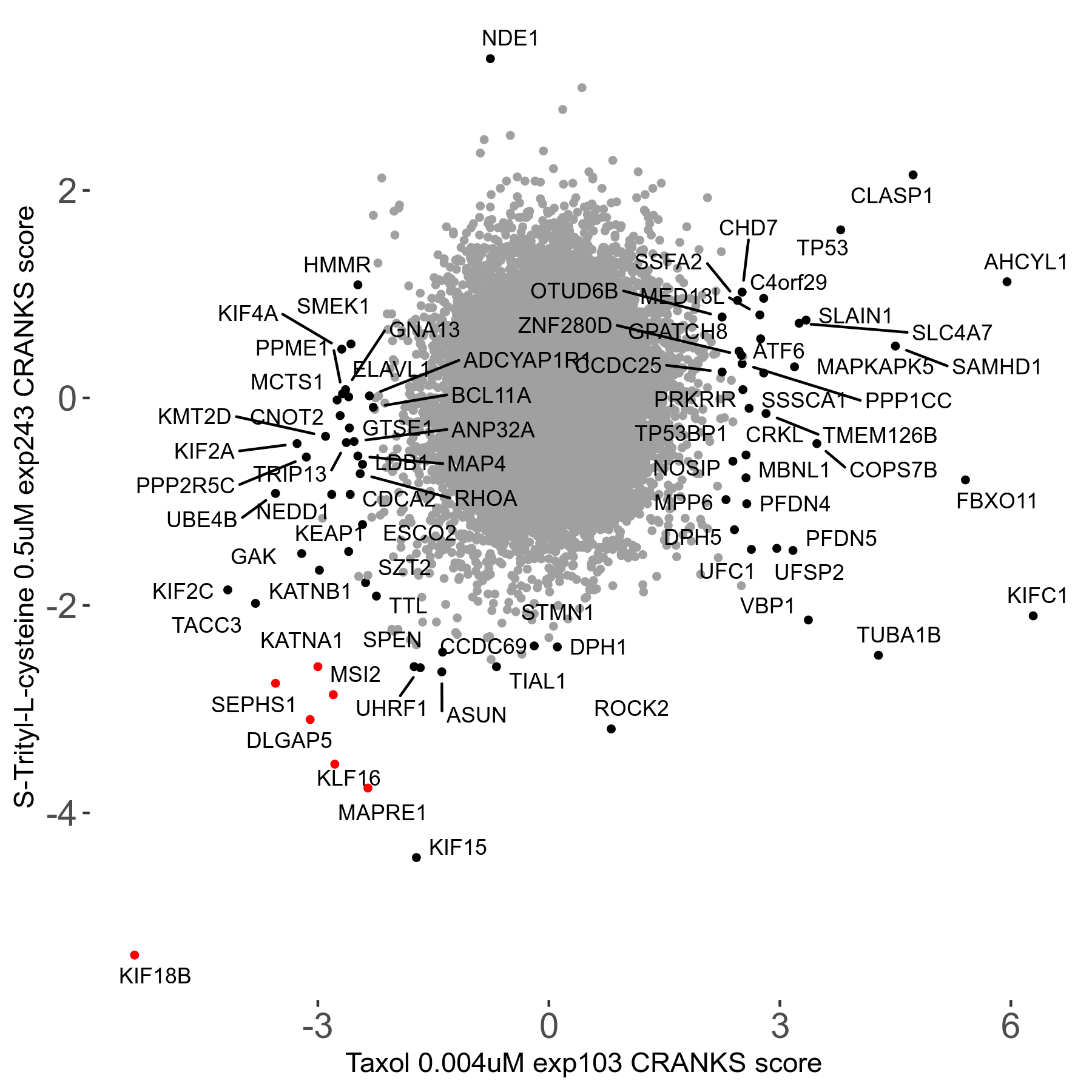
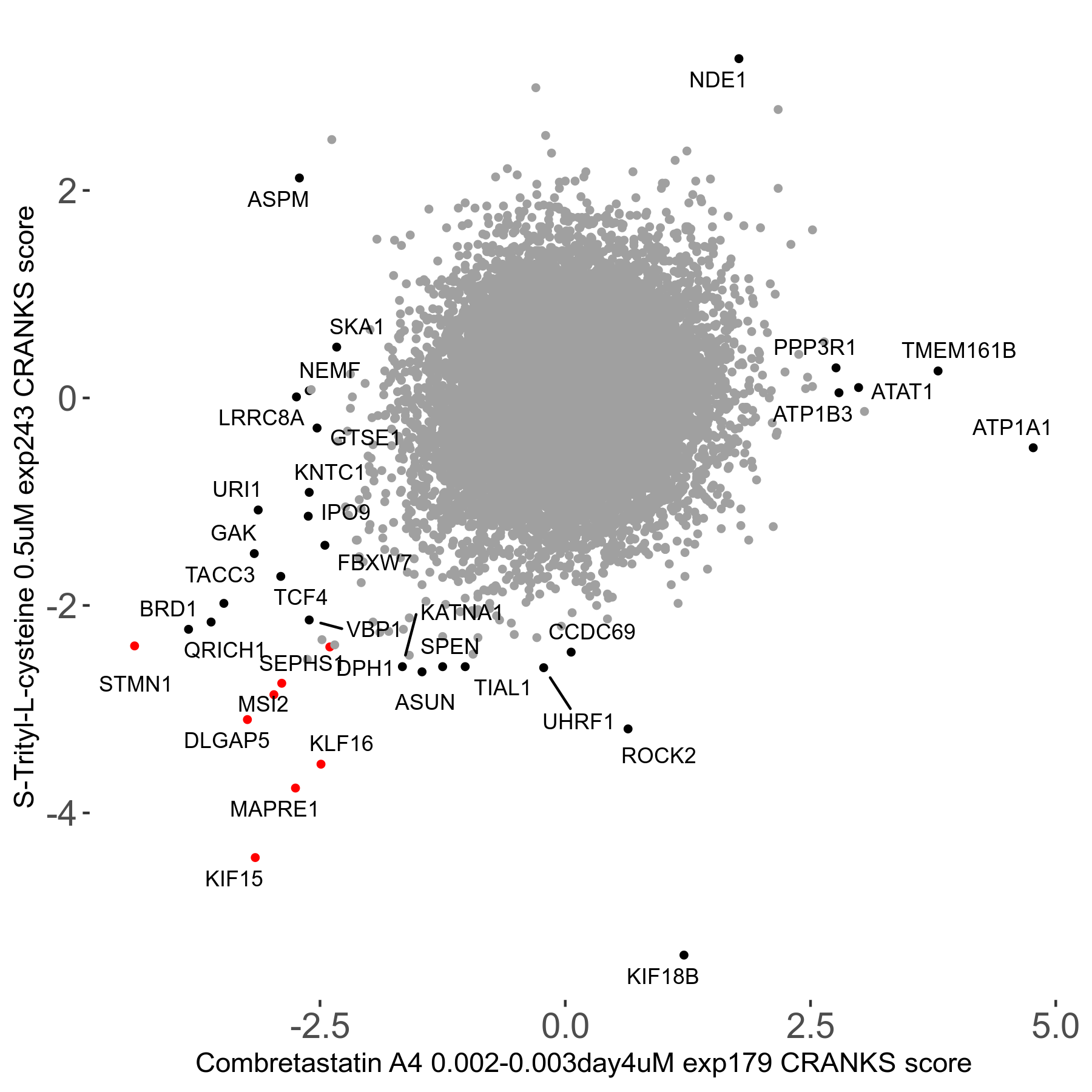
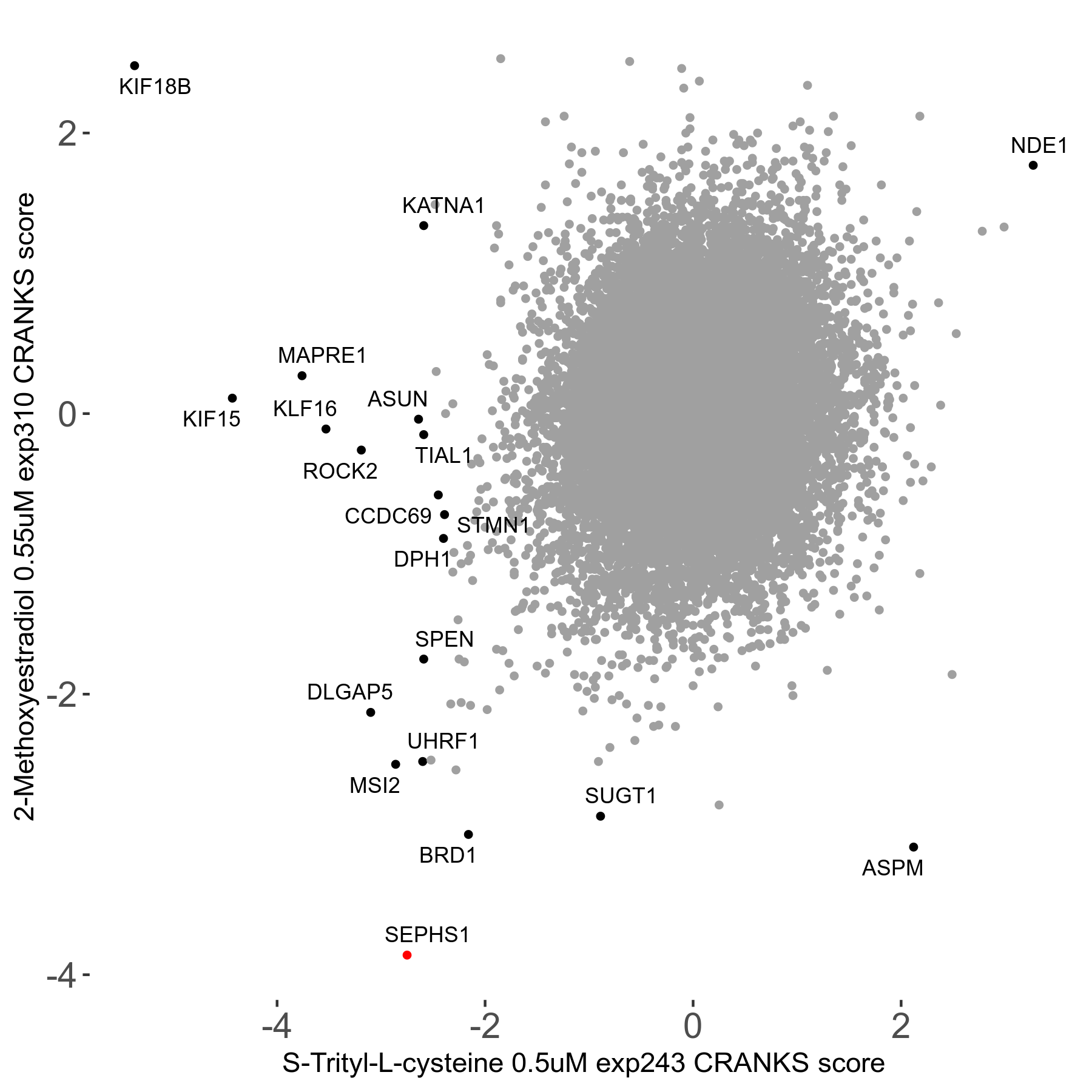
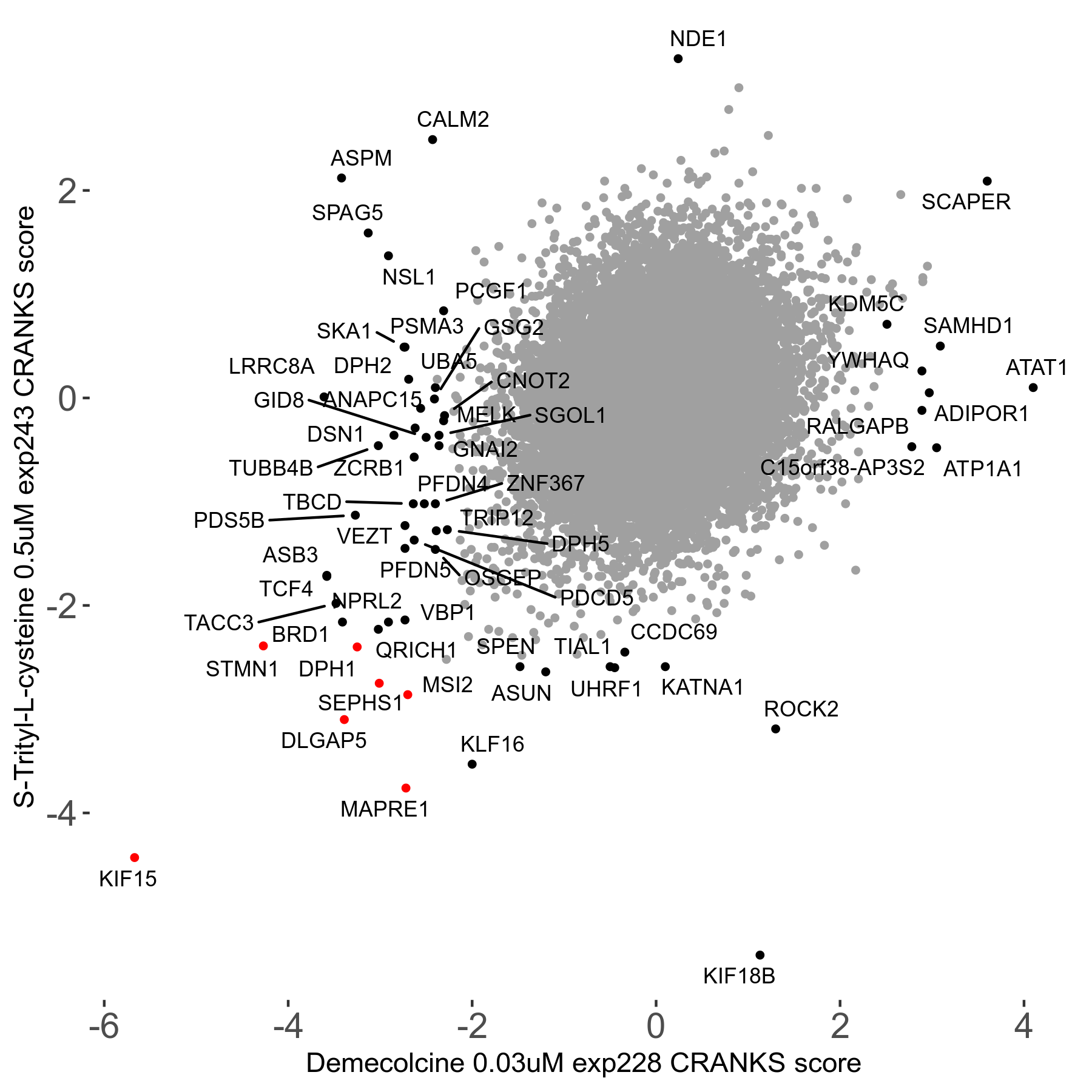
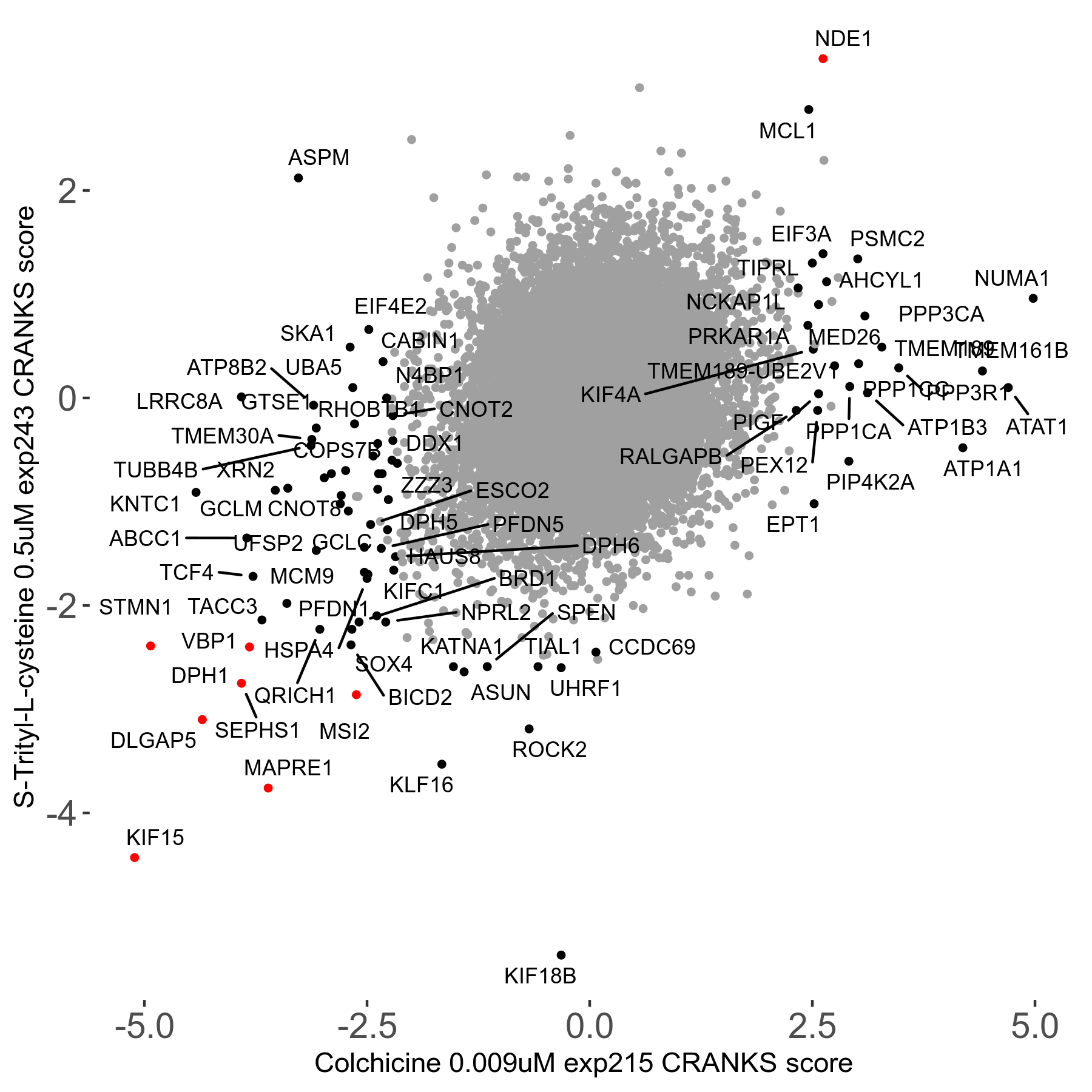
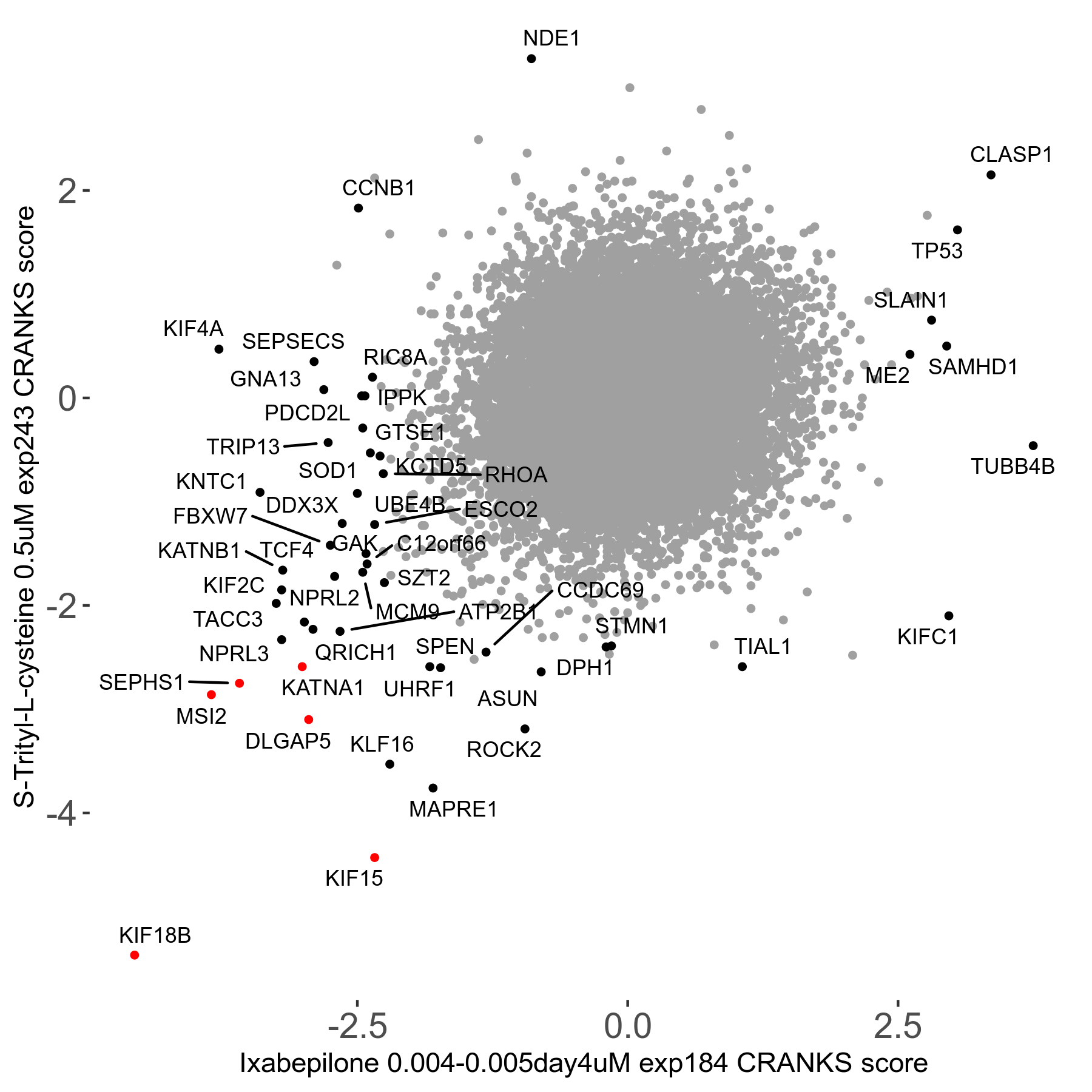
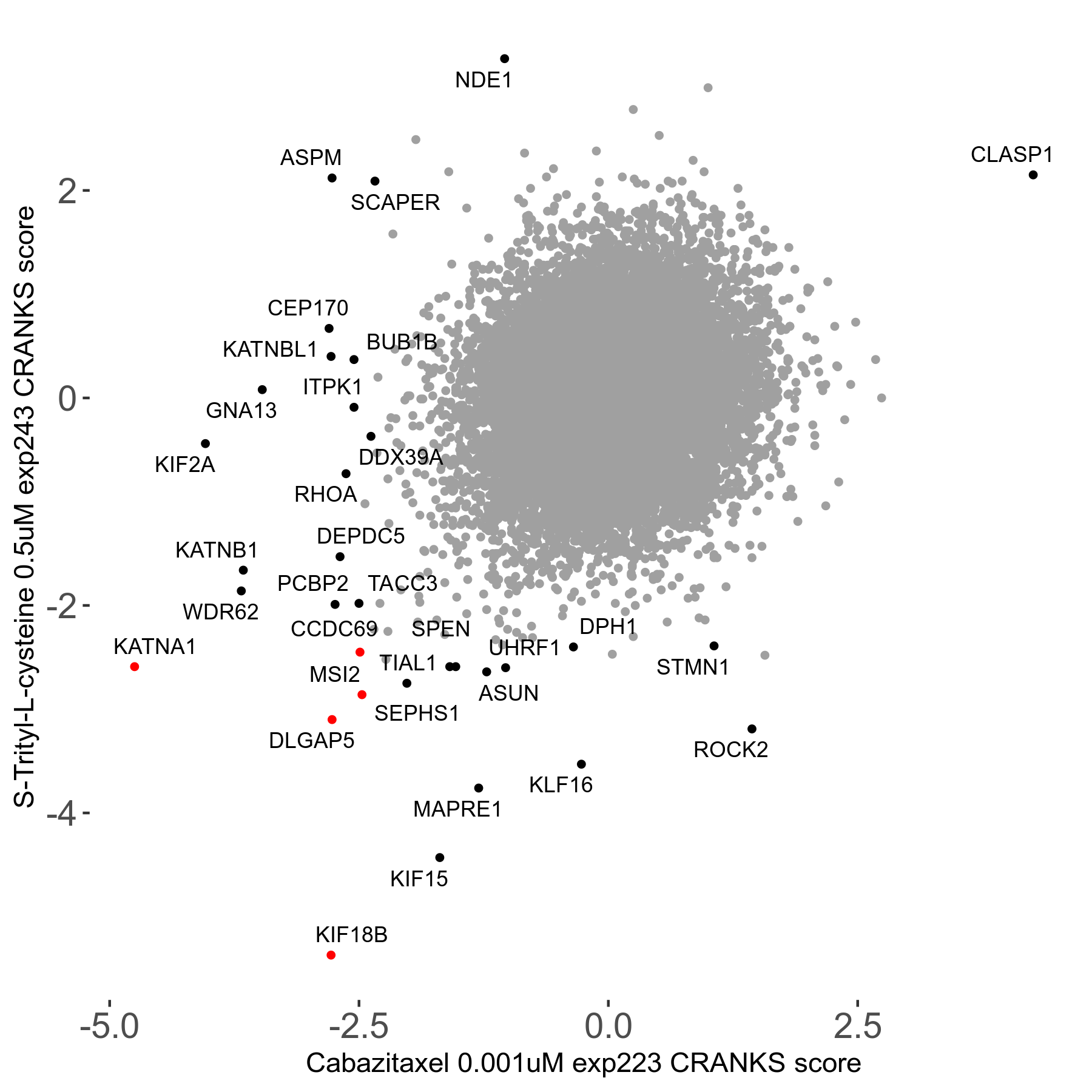
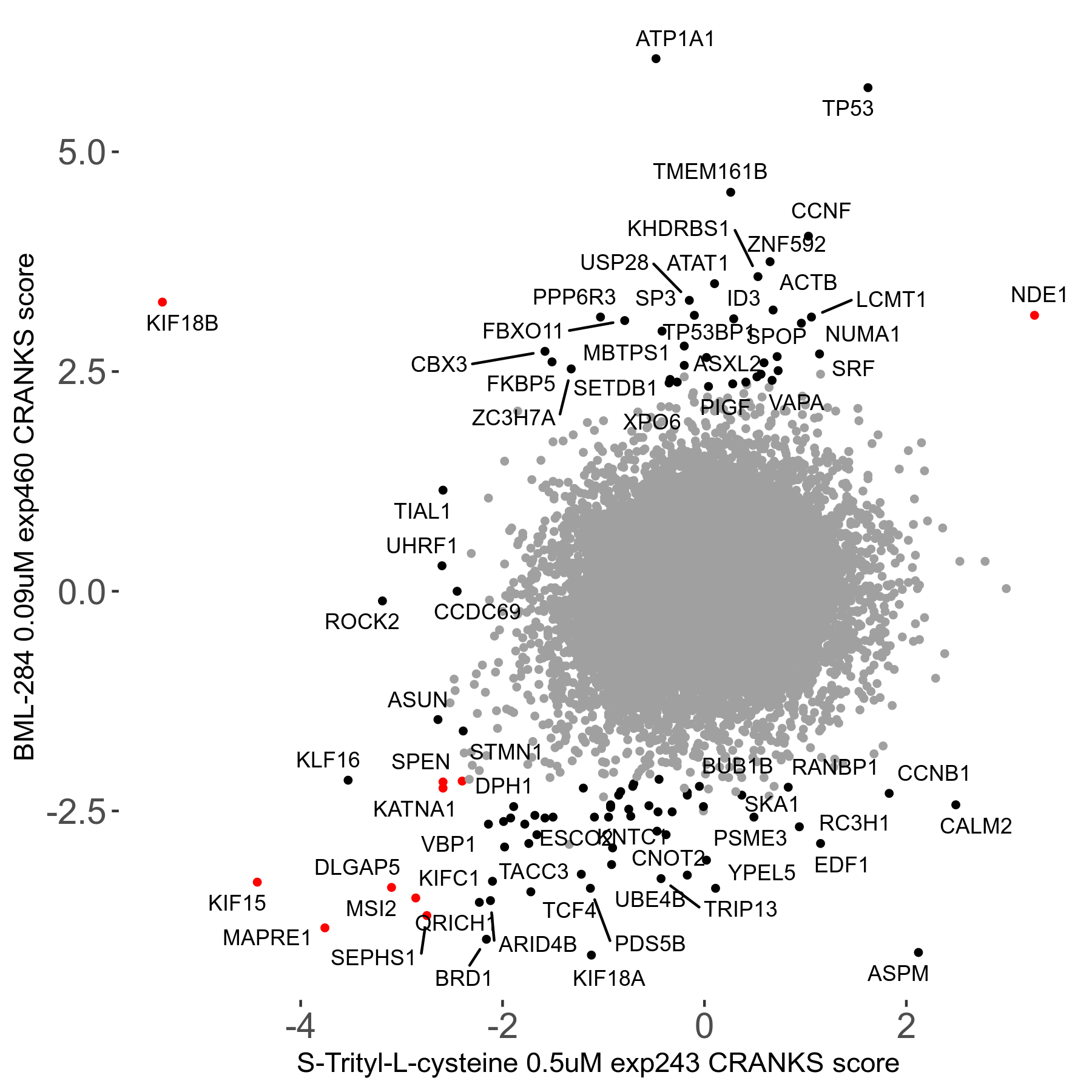
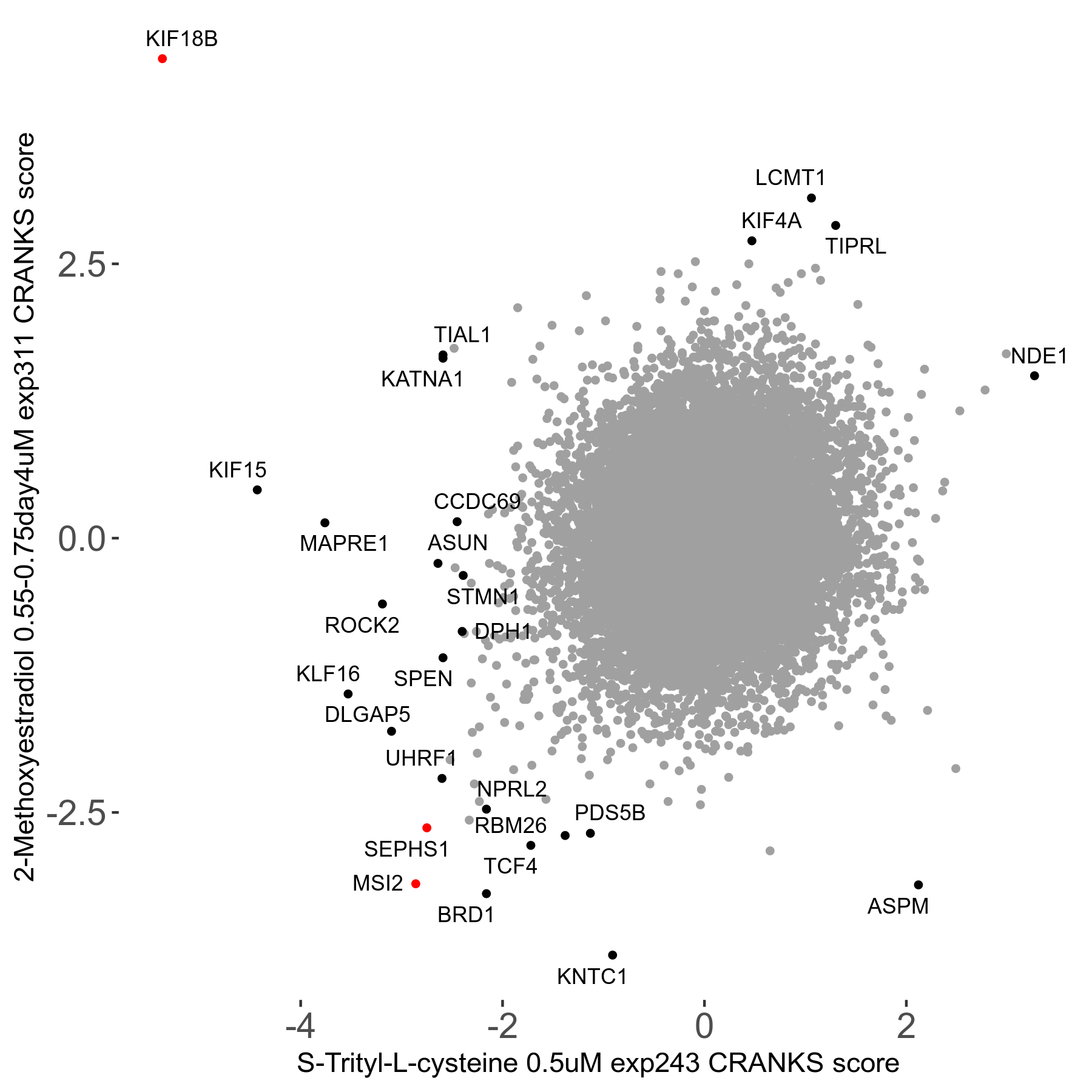
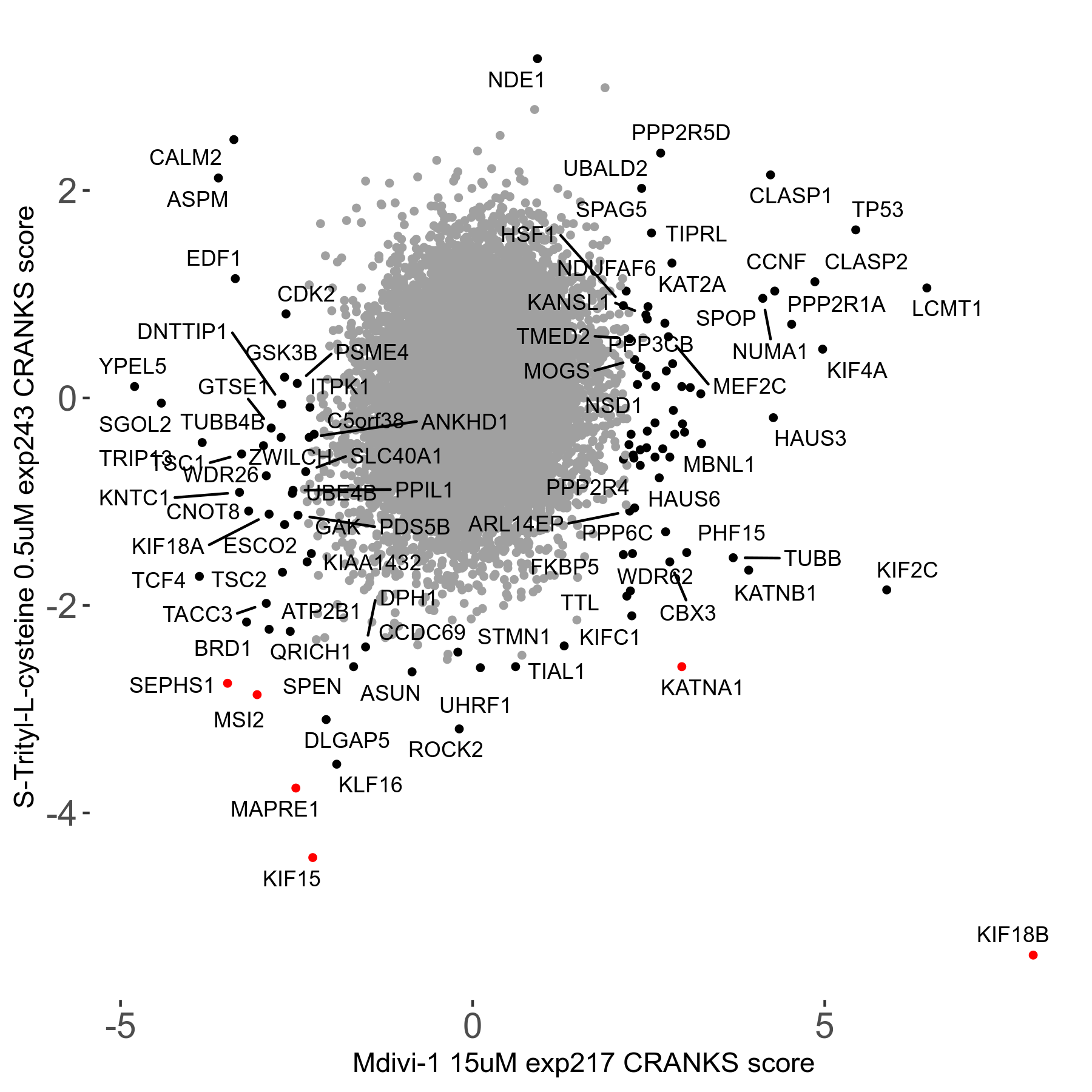
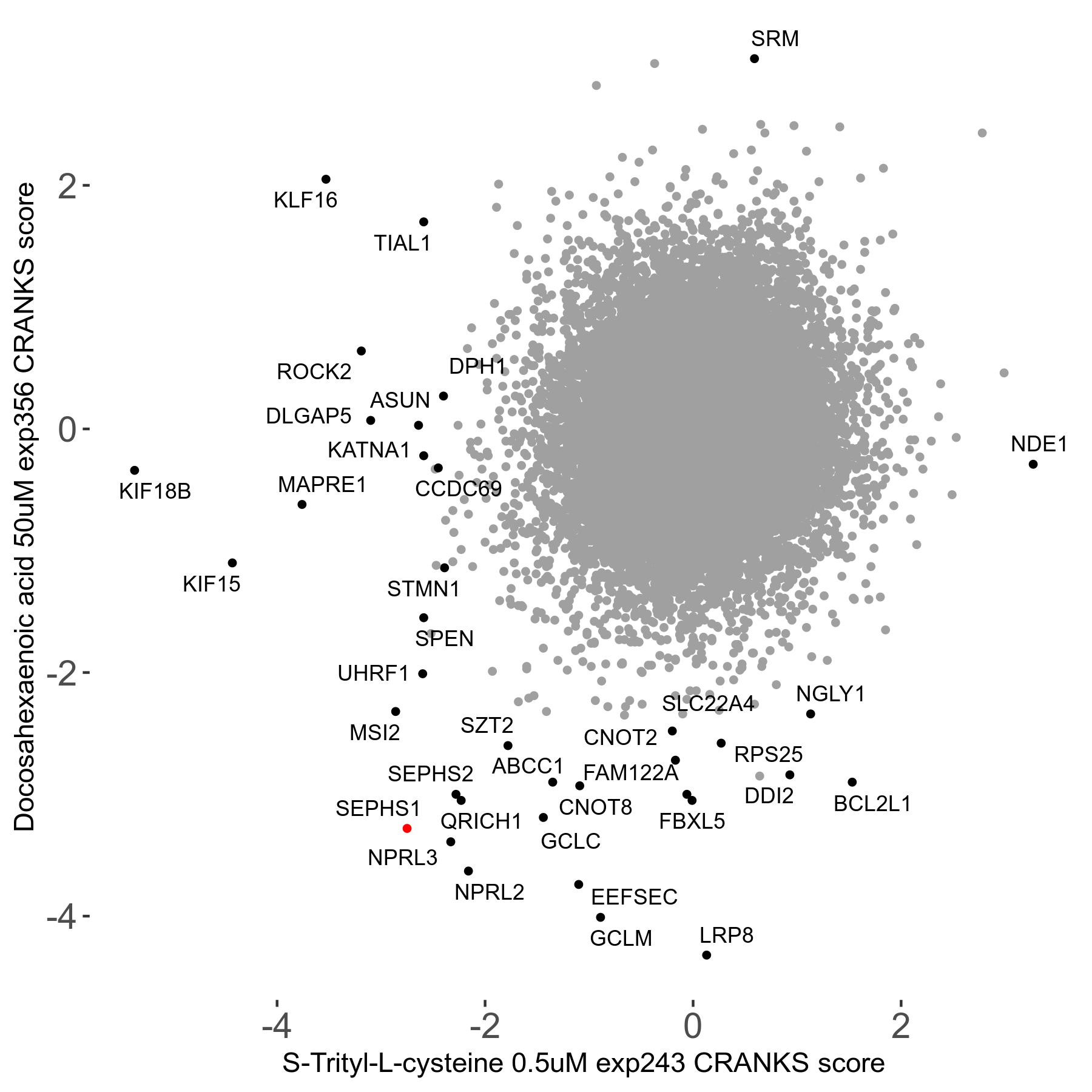
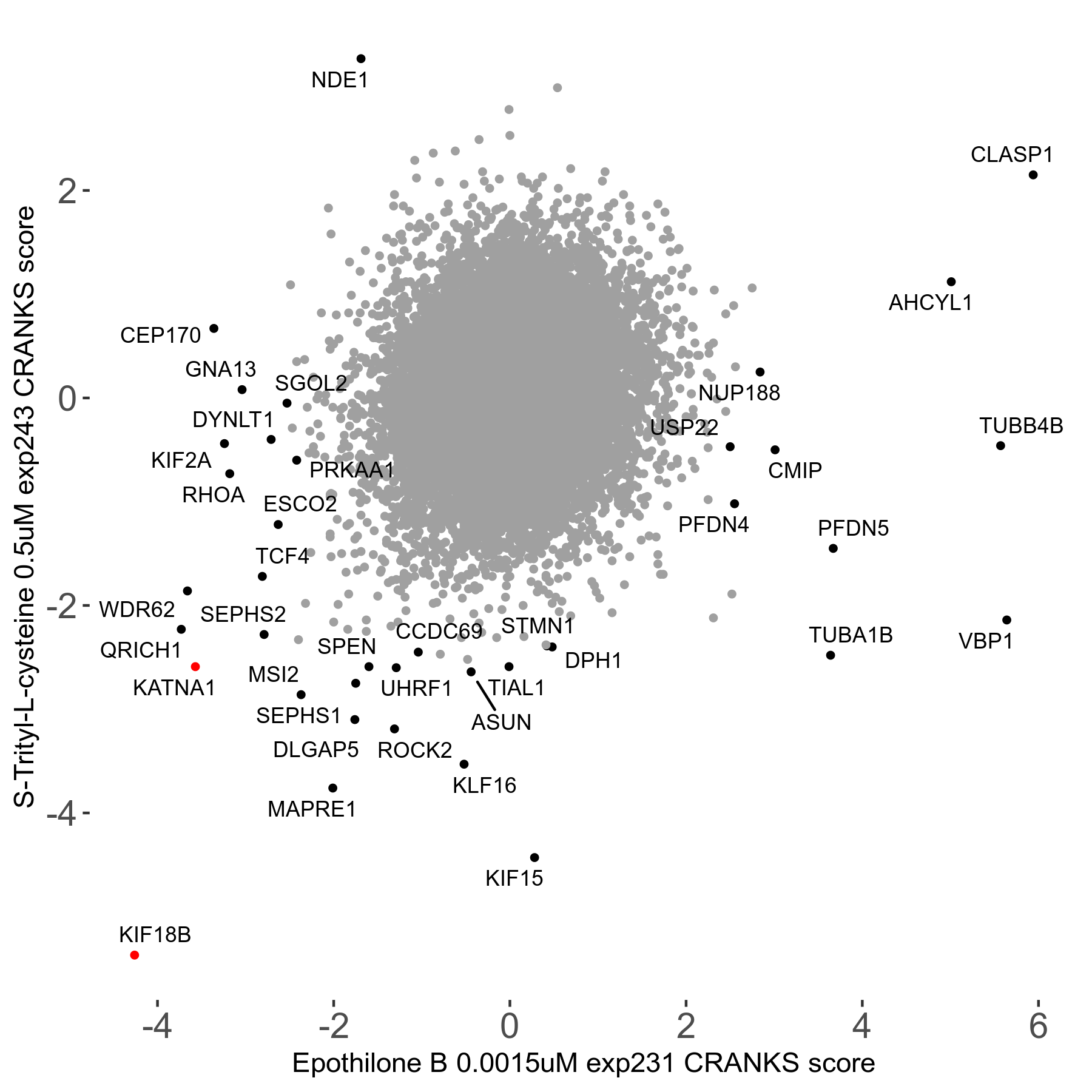
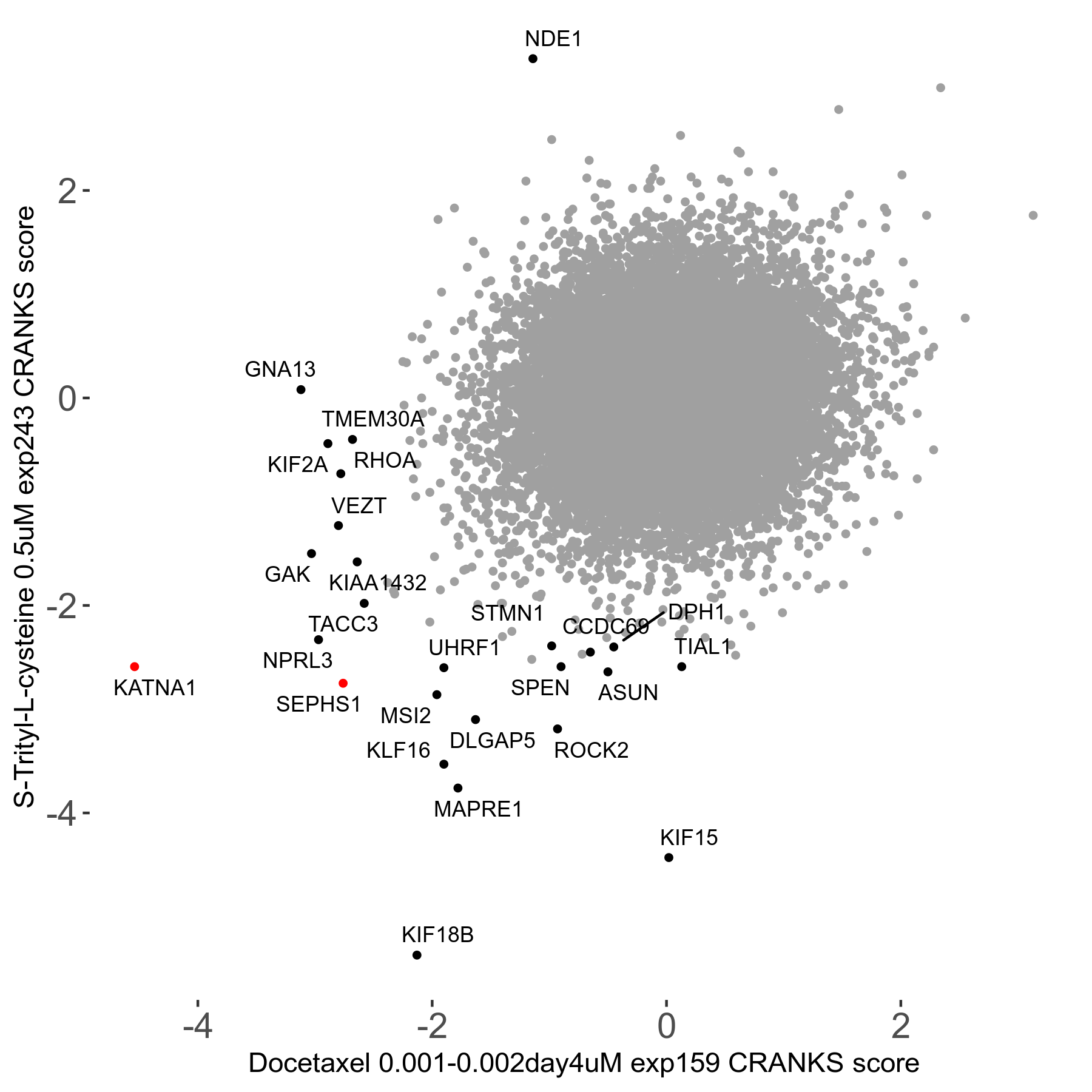
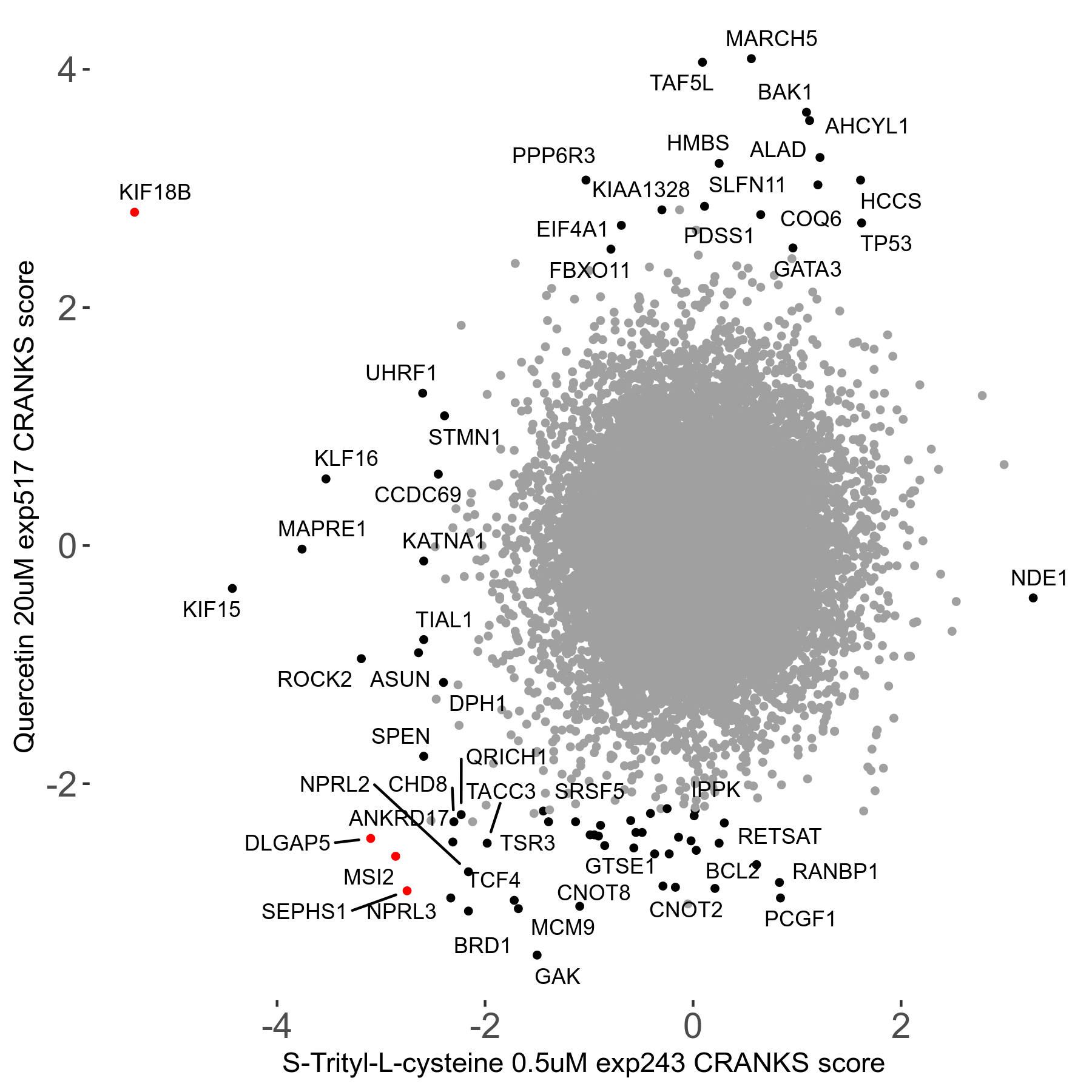
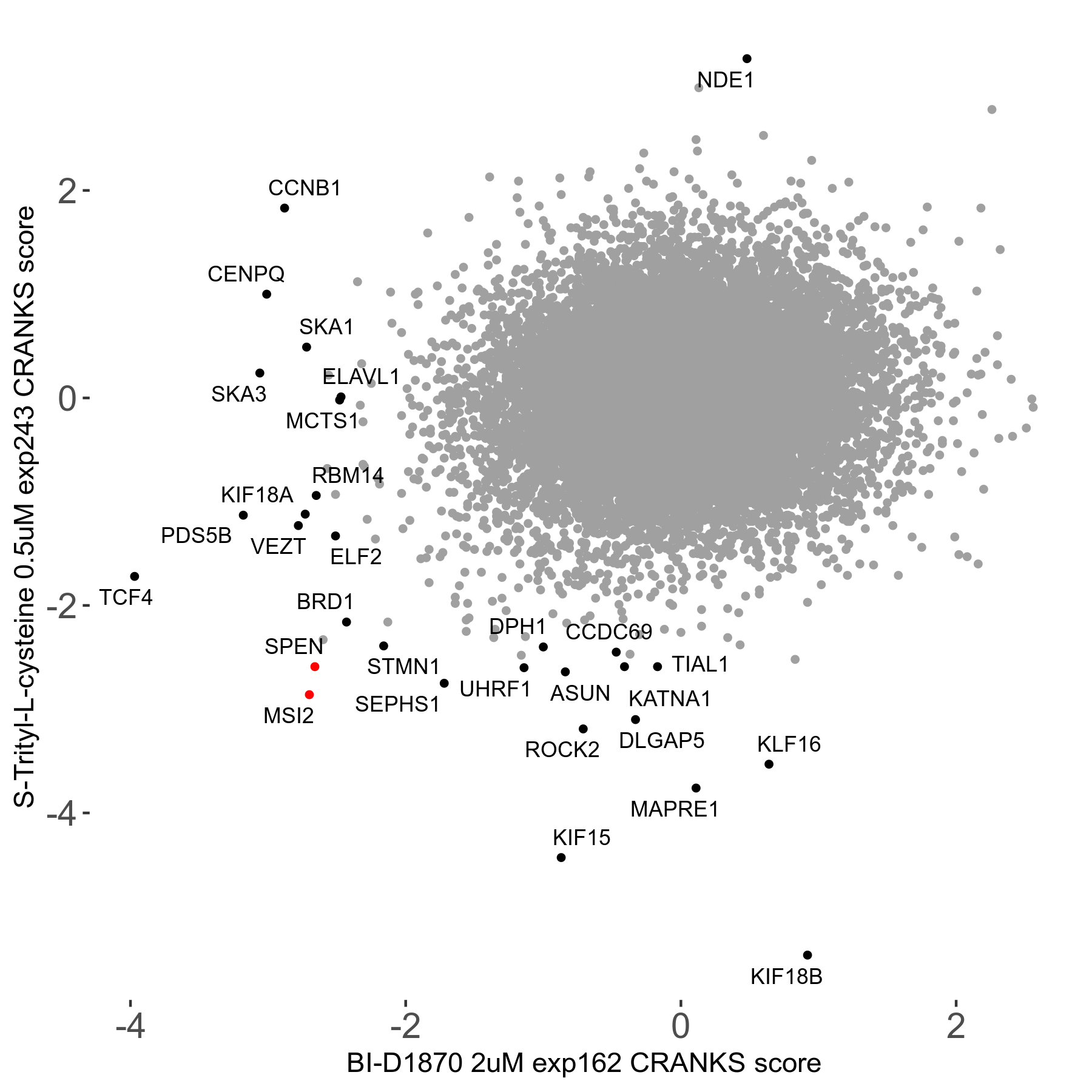
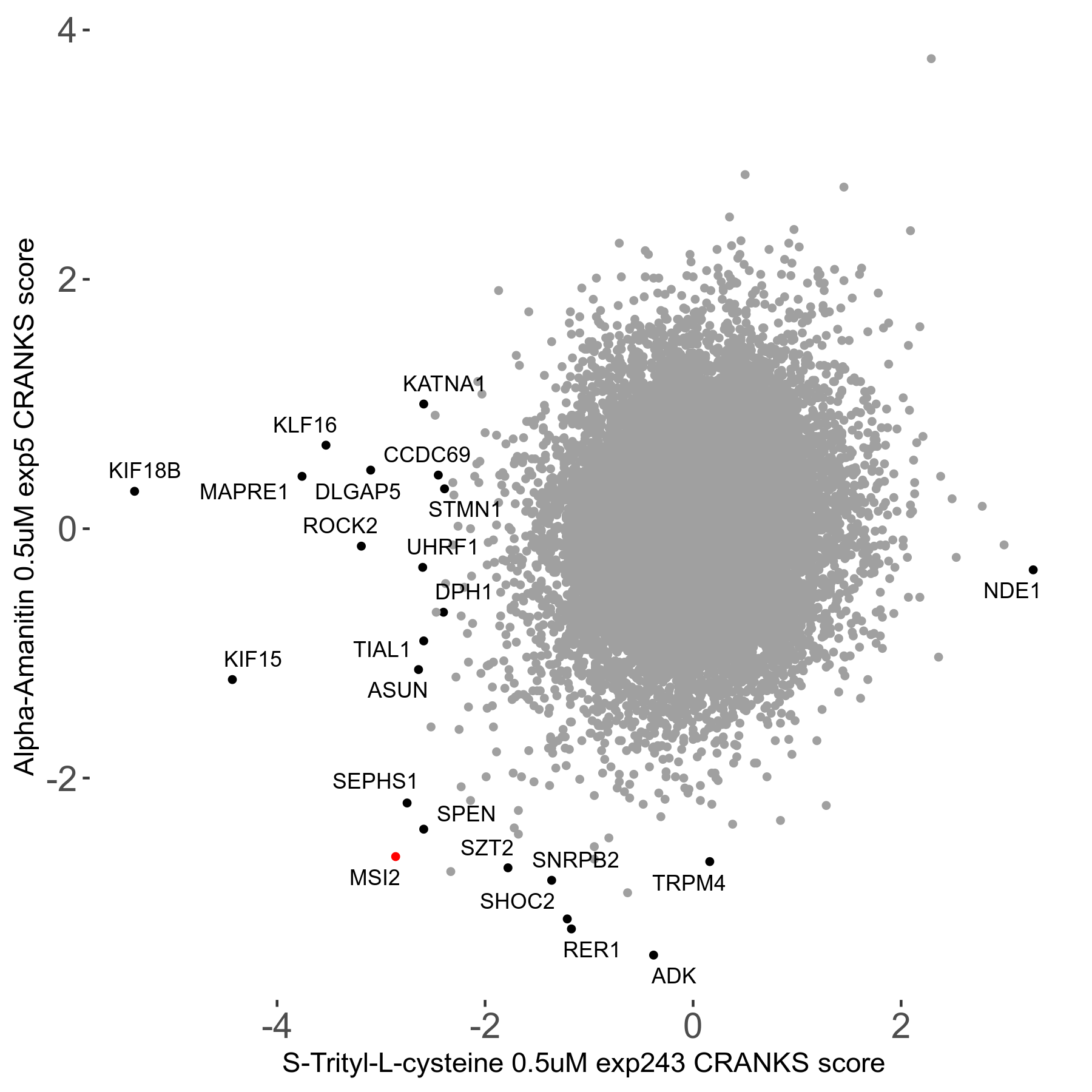
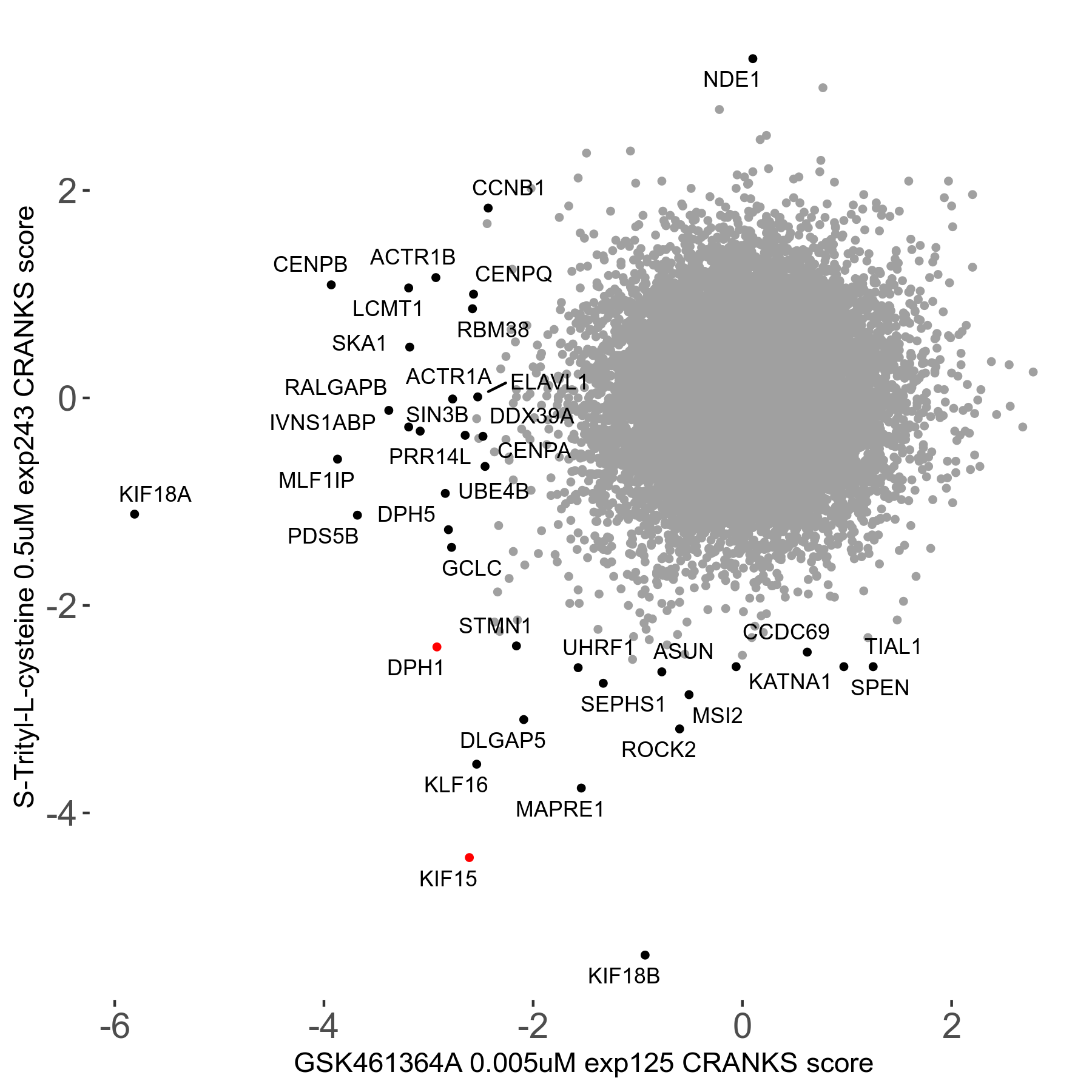
S-trityl-L-cysteine 0.5μM R05 exp243
Mechanism of Action
Inhibits Eg5 kinesin, blocks centrosome separation and bipolar spindle formation, arrests cells with monoastral spindles
- Class / Subclass 1: Organelle Function / Cytoskeletal Inhibitor
- Class / Subclass 2: Cell Cycle / Microtubule Poison
Technical Notes
Compound References
- PubChem Name: S-Trityl-L-cysteine
- Synonyms: N/A
- CAS #: 2799-07-7
- PubChem CID: 76044
- IUPAC: (2R)-2-amino-3-tritylsulfanylpropanoic acid
- INCHI Name: InChI=1S/C22H21NO2S/c23-20(21(24)25)16-26-22(17-10-4-1-5-11-17,18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-15,20H,16,23H2,(H,24,25)/t20-/m0/s1
- INCHI Key: DLMYFMLKORXJPO-FQEVSTJZSA-N
- Molecular Weight: 363.5
- Canonical SMILES: C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)SCC(C(=O)O)N
- Isomeric SMILES: C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)SC[C@@H](C(=O)O)N
- Molecular Formula: C22H21NO2S
Compound Supplier
- Supplier Name: FOCUS Biomolecules
- Catalog #: 10-1042
- Lot #: N/A
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C22H21NO2S 386.11852; found 386.11917
Dose Response Curve
- Platform ID: STLC
- Min: -14.0321; Max: 86.9900

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 0.3654 |
| IC30 | 0.4628 |
| IC40 | 0.5618 |
| IC50 | 0.6711 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 05
- Dose: 500nM
- Days of incubation: 8
- Doublings: 6.9
- Numbers of reads: 21702335
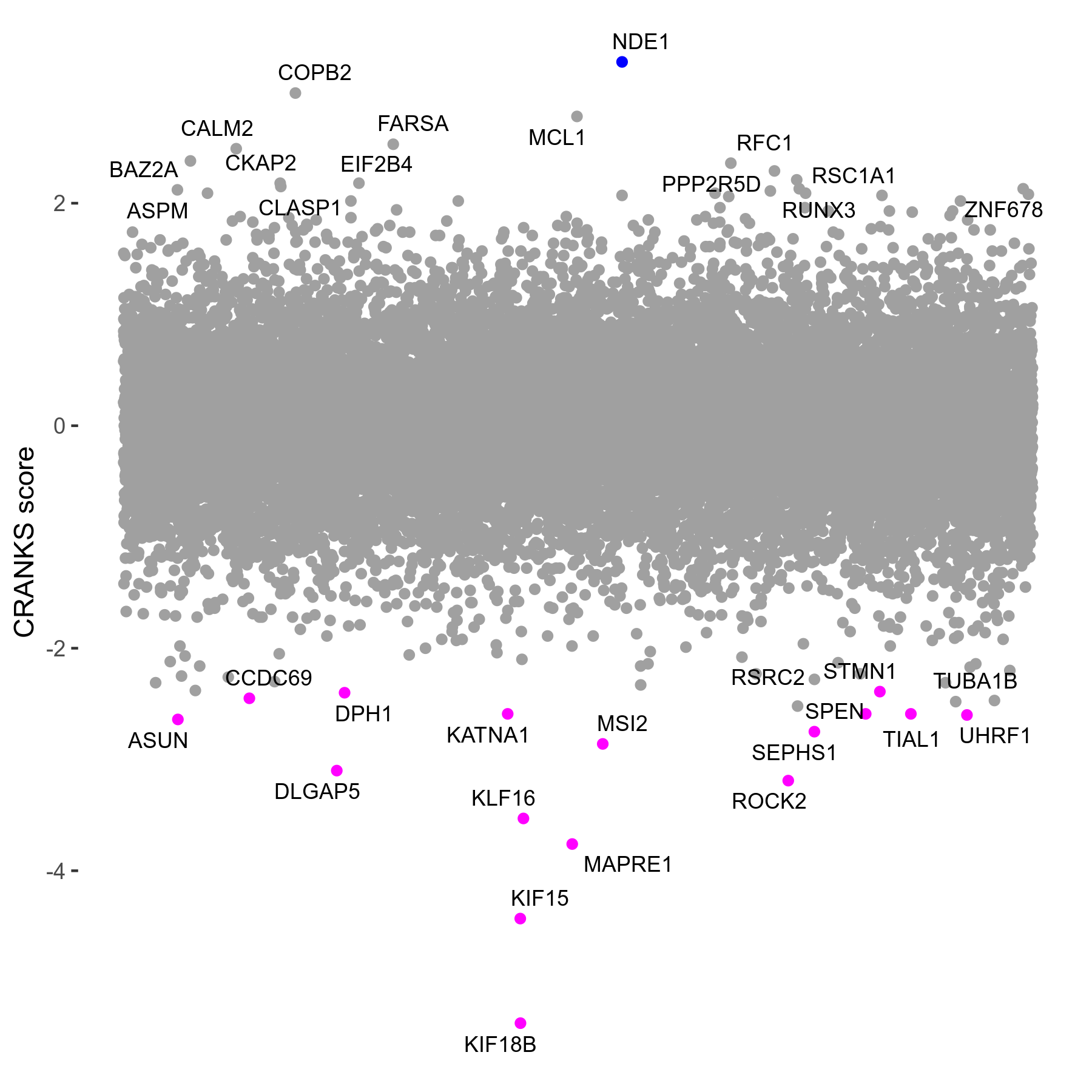
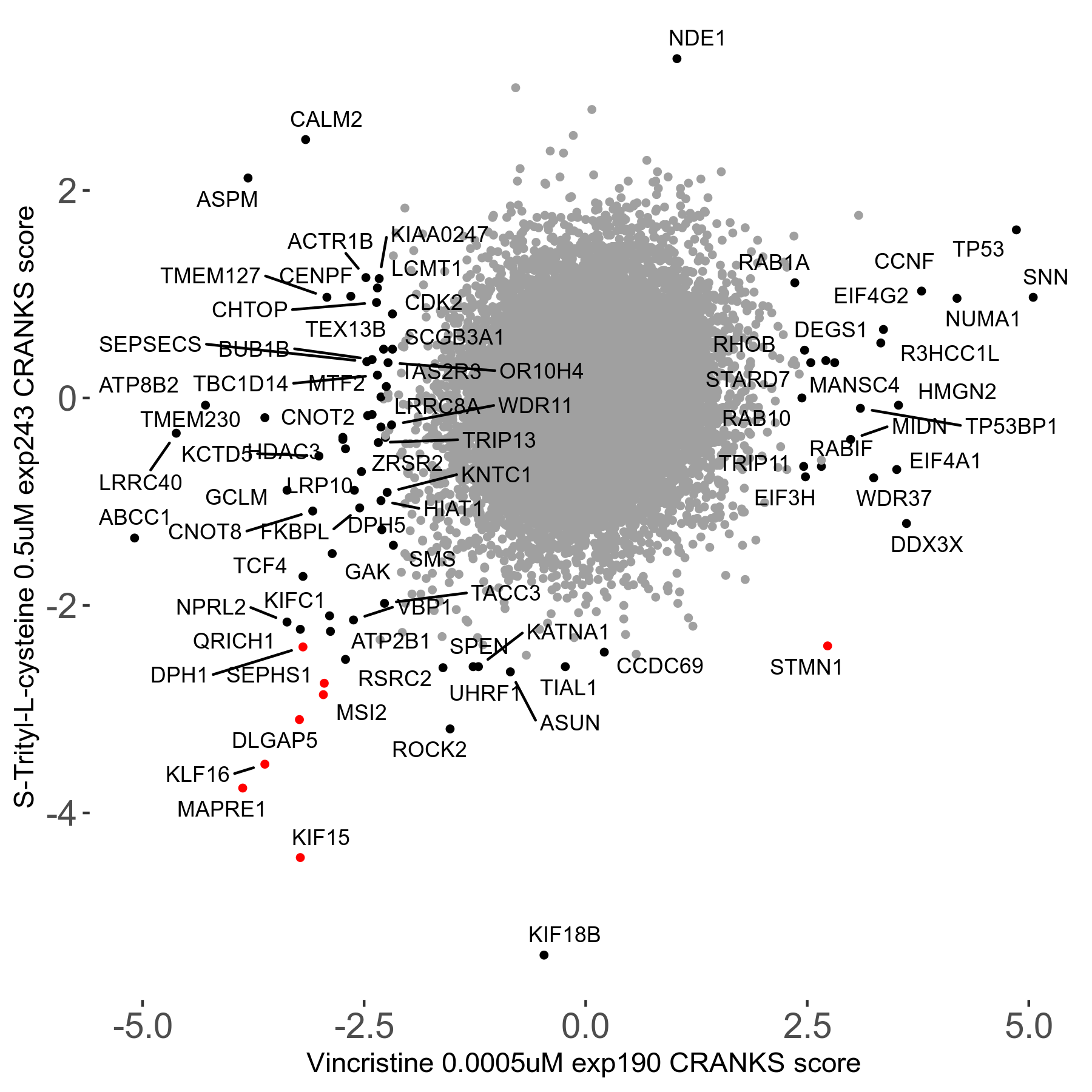
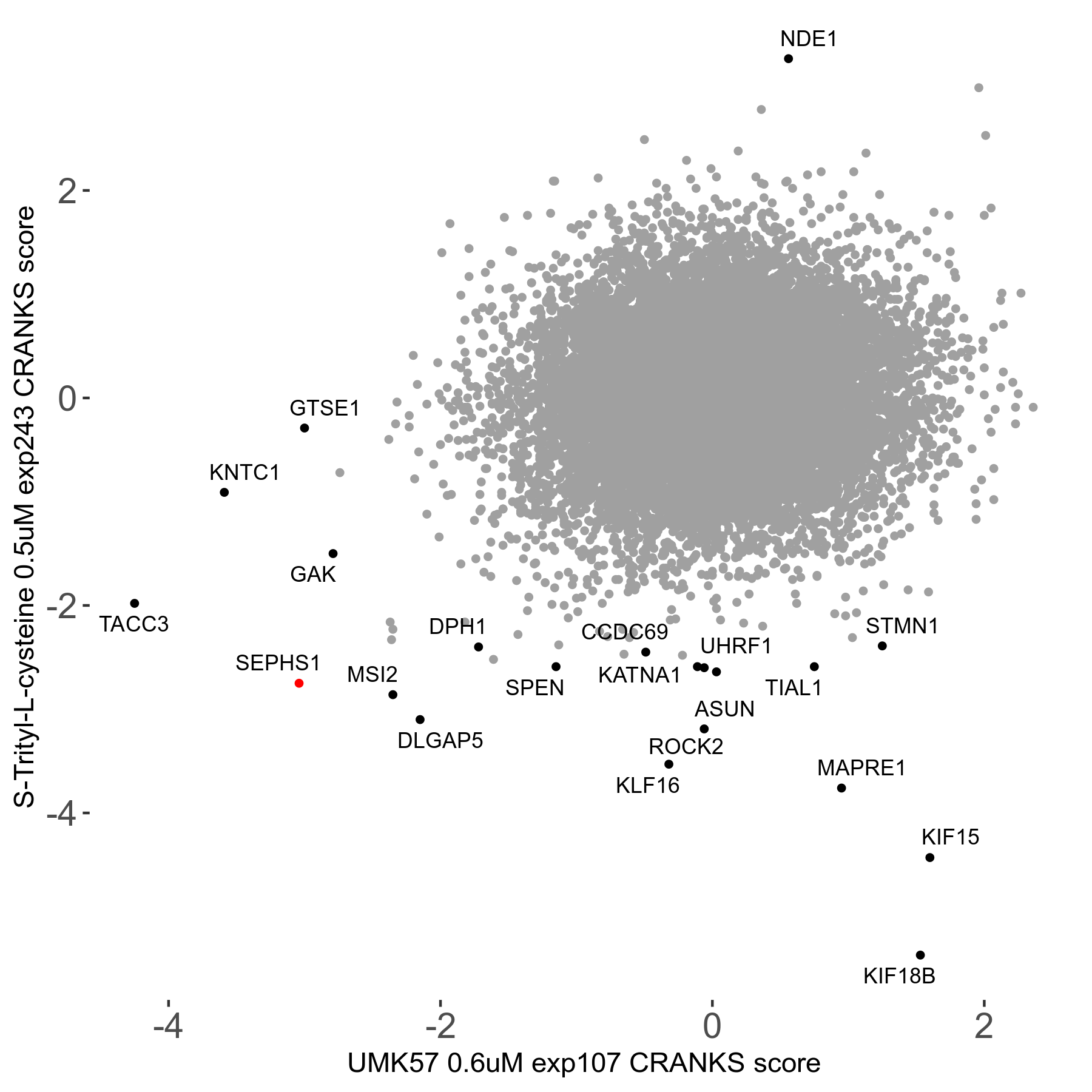
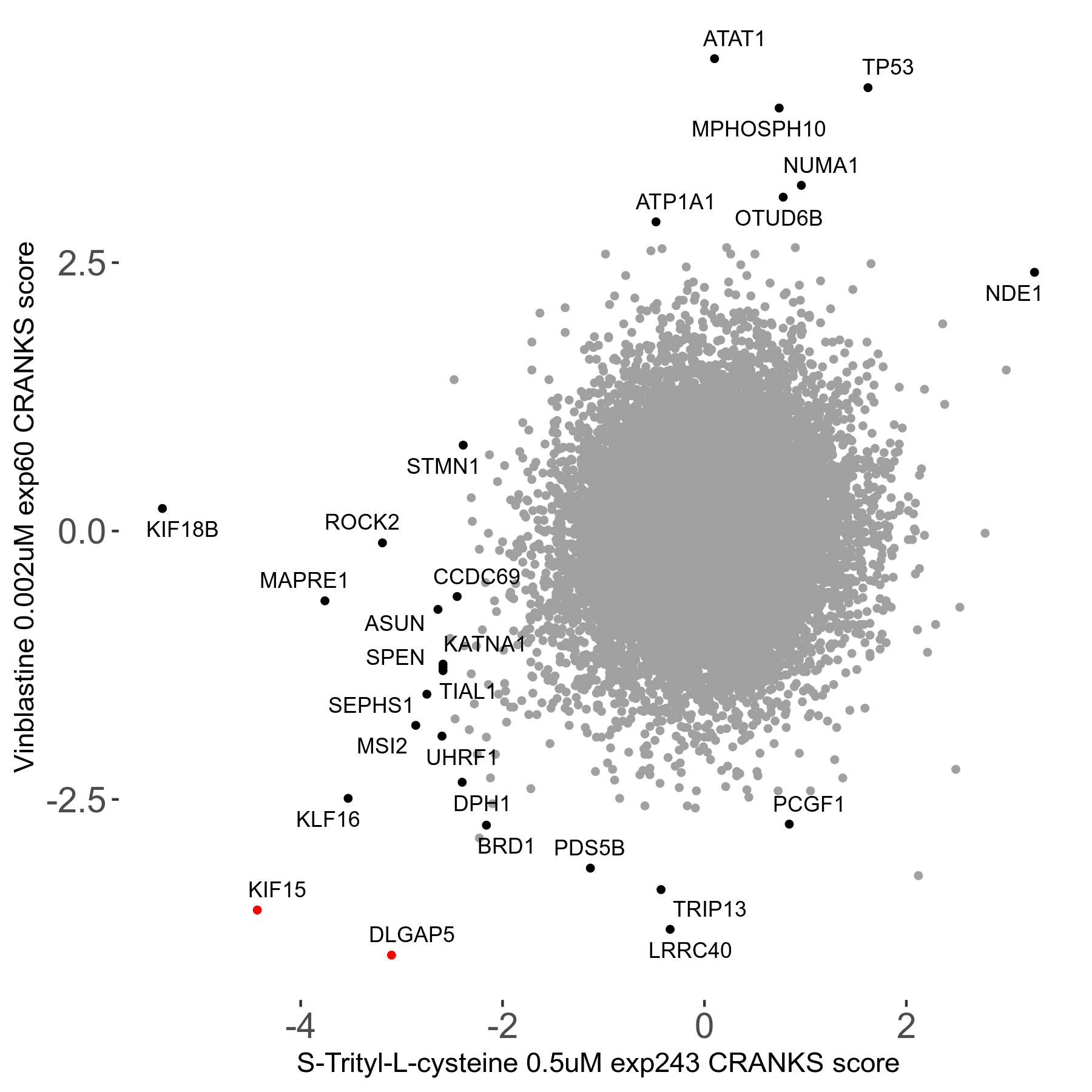
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 16/1 | Scores |