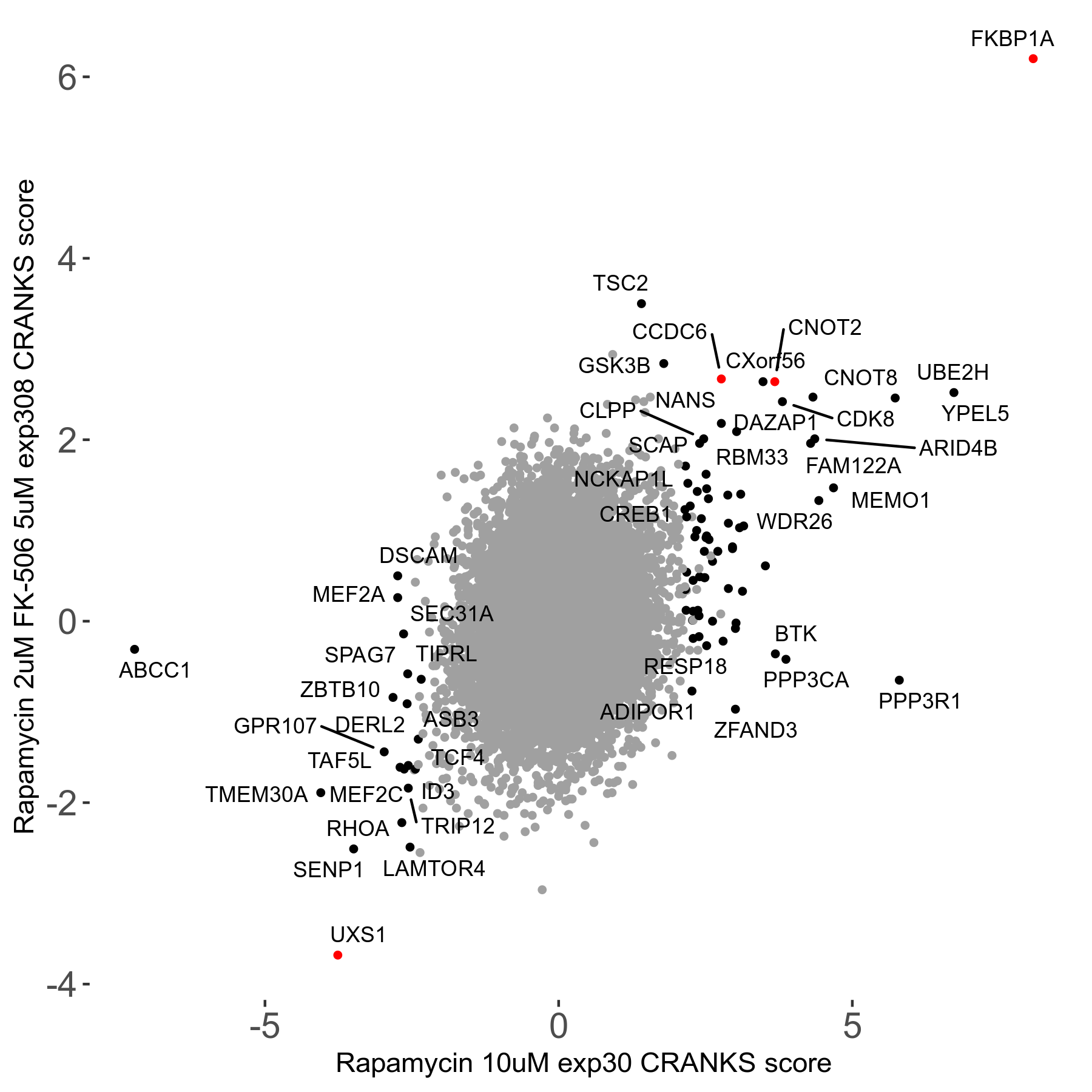
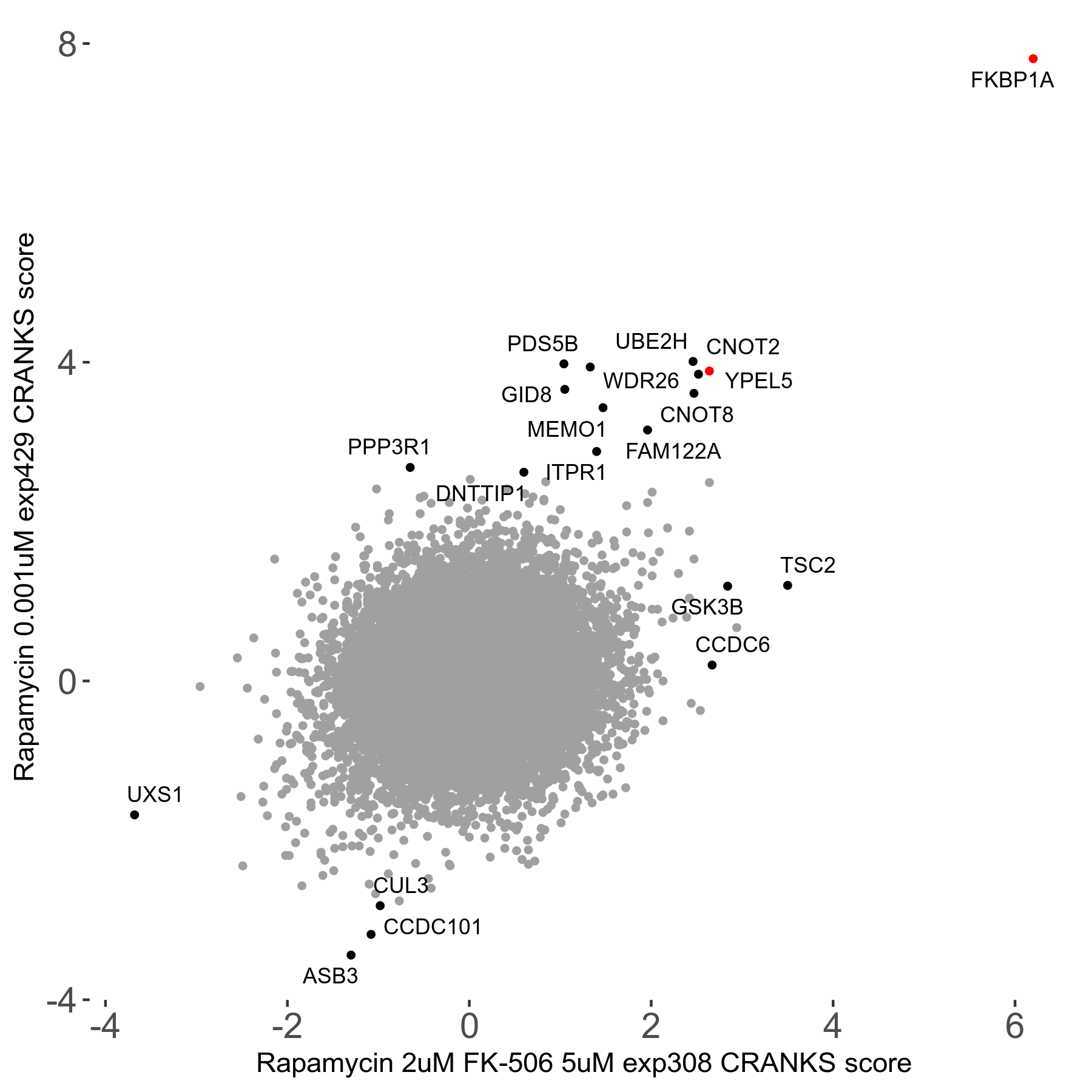
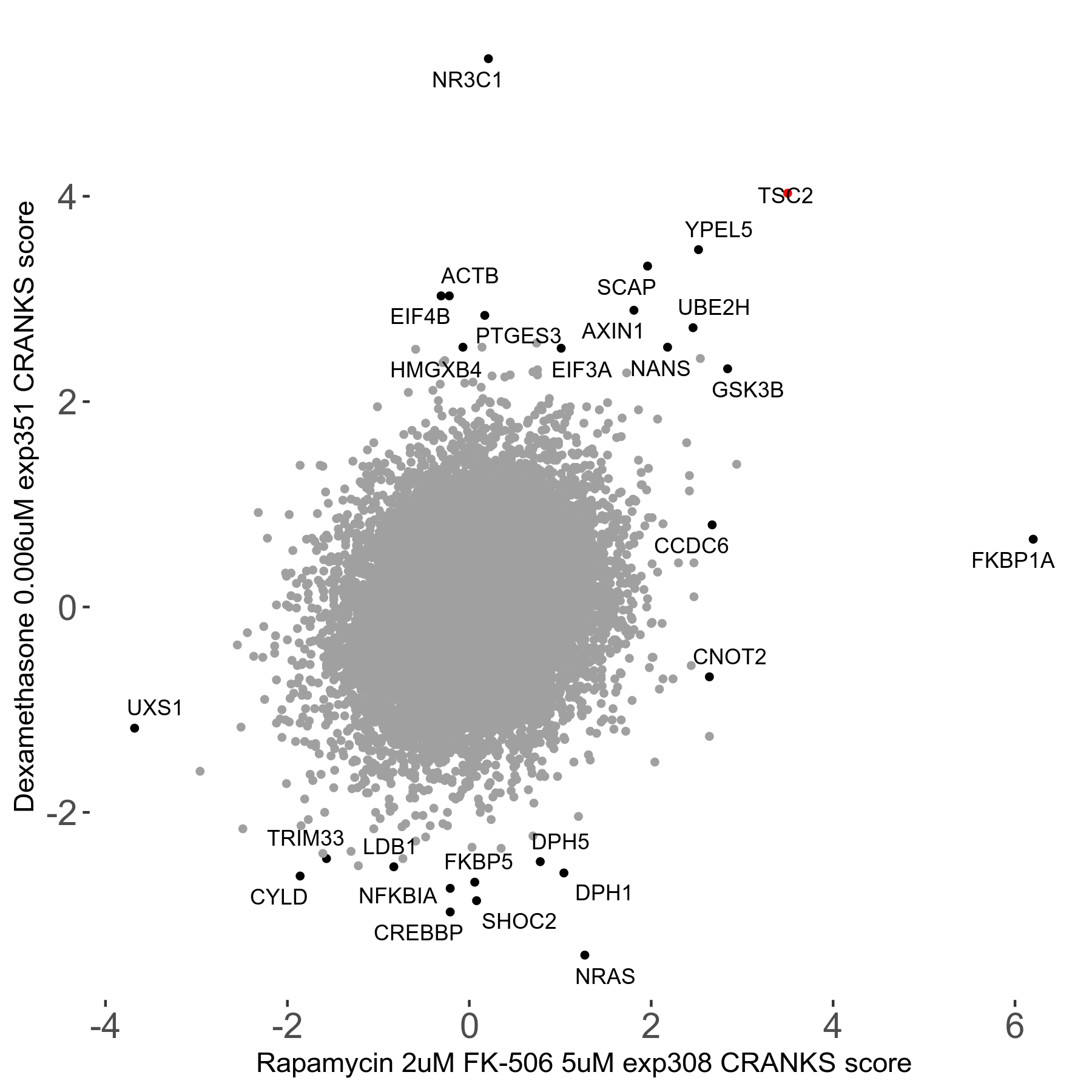
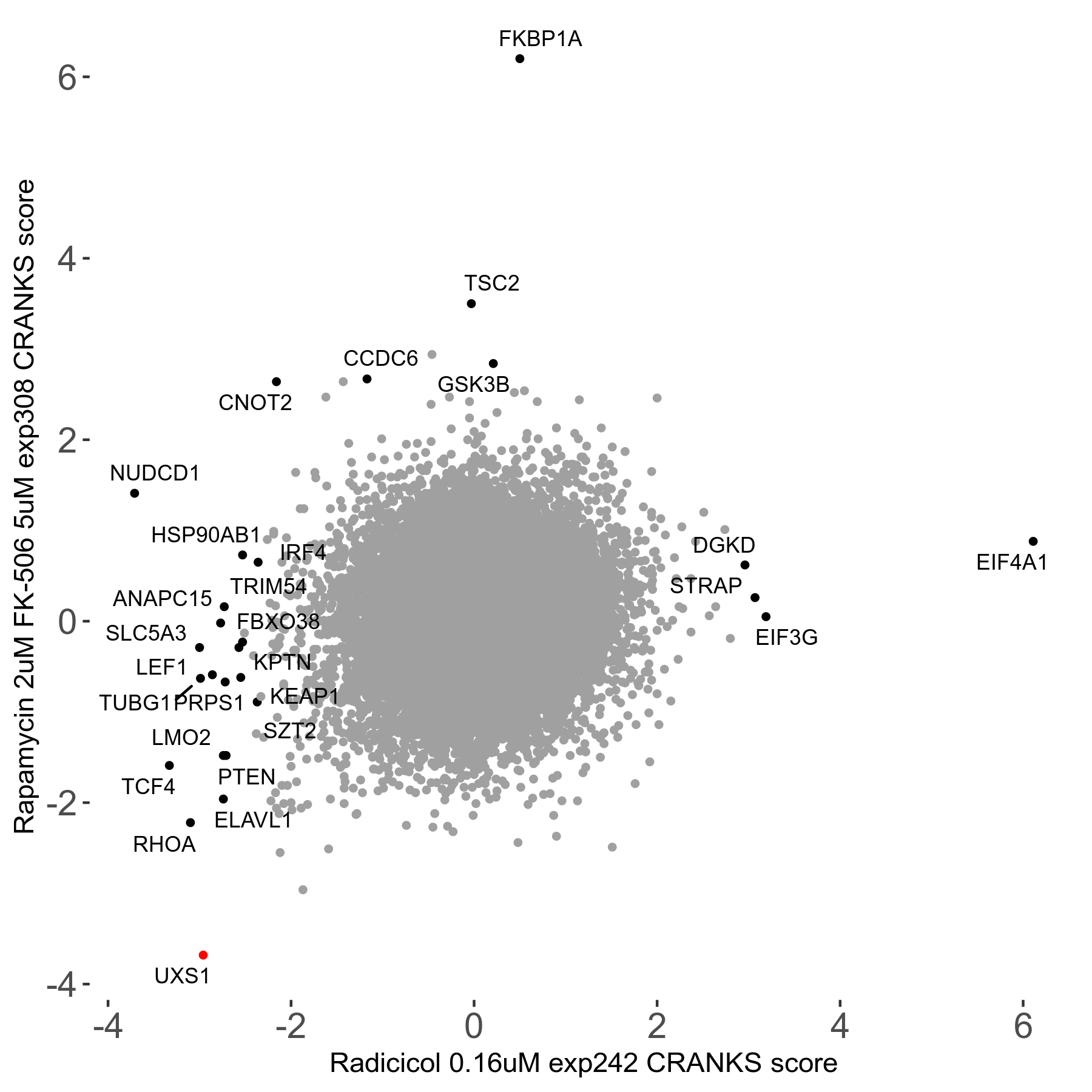
Rapamycin 2μM plus FK-506 5μM R07 exp308
Mechanism of Action
Allosteric mTORC1 inhibitor, forms ternary complex with FKBP12 and mTOR, attenuates protein synthesis and metabolism
- Class / Subclass 1: Signal Transduction / Kinase Inhibitor
- Class / Subclass 2: Proteostasis / mTOR inhibitor
Technical Notes
Rapamycin
Compound References
- PubChem Name: Sirolimus
- Synonyms: Sirolimus; AY 22989
- CAS #: 53123-88-9
- PubChem CID: 5284616
- IUPAC: (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
- INCHI Name: InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
- INCHI Key: QFJCIRLUMZQUOT-HPLJOQBZSA-N
- Molecular Weight: 914.2
- Canonical SMILES: CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)O)C)C)O)OC)C)C)C)OC
- Isomeric SMILES: C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)O)C)/C)O)OC)C)C)/C)OC
- Molecular Formula: C51H79NO13
Compound Supplier
- Supplier Name: LC Laboratories
- Catalog #: R-5000
- Lot #: ASW-130
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C51H79NO13 936.54436; found 936.54366
FK-506
Compound References
- PubChem Name: Tacrolimus
- Synonyms: FK506; Fujimycin; FR900506
- CAS #: 104987-11-3
- PubChem CID: 445643
- IUPAC: (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-1,14-dihydroxy-12-[(E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-17-prop-2-enyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone
- INCHI Name: InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19+,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1
- INCHI Key: QJJXYPPXXYFBGM-LFZNUXCKSA-N
- Molecular Weight: 804
- Canonical SMILES: CC1CC(C2C(CC(C(O2)(C(=O)C(=O)N3CCCCC3C(=O)OC(C(C(CC(=O)C(C=C(C1)C)CC=C)O)C)C(=CC4CCC(C(C4)OC)O)C)O)C)OC)OC
- Isomeric SMILES: C[C@@H]1C[C@@H]([C@@H]2[C@H](C[C@H]([C@@](O2)(C(=O)C(=O)N3CCCC[C@H]3C(=O)O[C@@H]([C@@H]([C@H](CC(=O)[C@@H](/C=C(/C1)\\C)CC=C)O)C)/C(=C/[C@@H]4CC[C@H]([C@@H](C4)OC)O)/C)O)C)OC)OC
- Molecular Formula: C44H69NO12
Compound Supplier
- Supplier Name: Toronto Research Chemicals
- Catalog #: F370000
- Lot #: N/A
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C44H69NO12 826.4712; found 826.47365
Rapamycin+FK-506
Dose Response Curve
- Platform ID: FK-506_5uM_Rapamycin
- Min: -29.7157; Max: 97.3162

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 8.9811 |
| IC30 | 10.1581 |
| IC40 | 11.4624 |
| IC50 | 12.9843 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
The dose response curve for Rapamycin is also available at:
The dose response curve for FK-506 is also available at:
Screen Summary
- Round: 07
- Dose: 2µM + 5µM
- Days of incubation: 8
- Doublings: 5.6
- Numbers of reads: 17119439
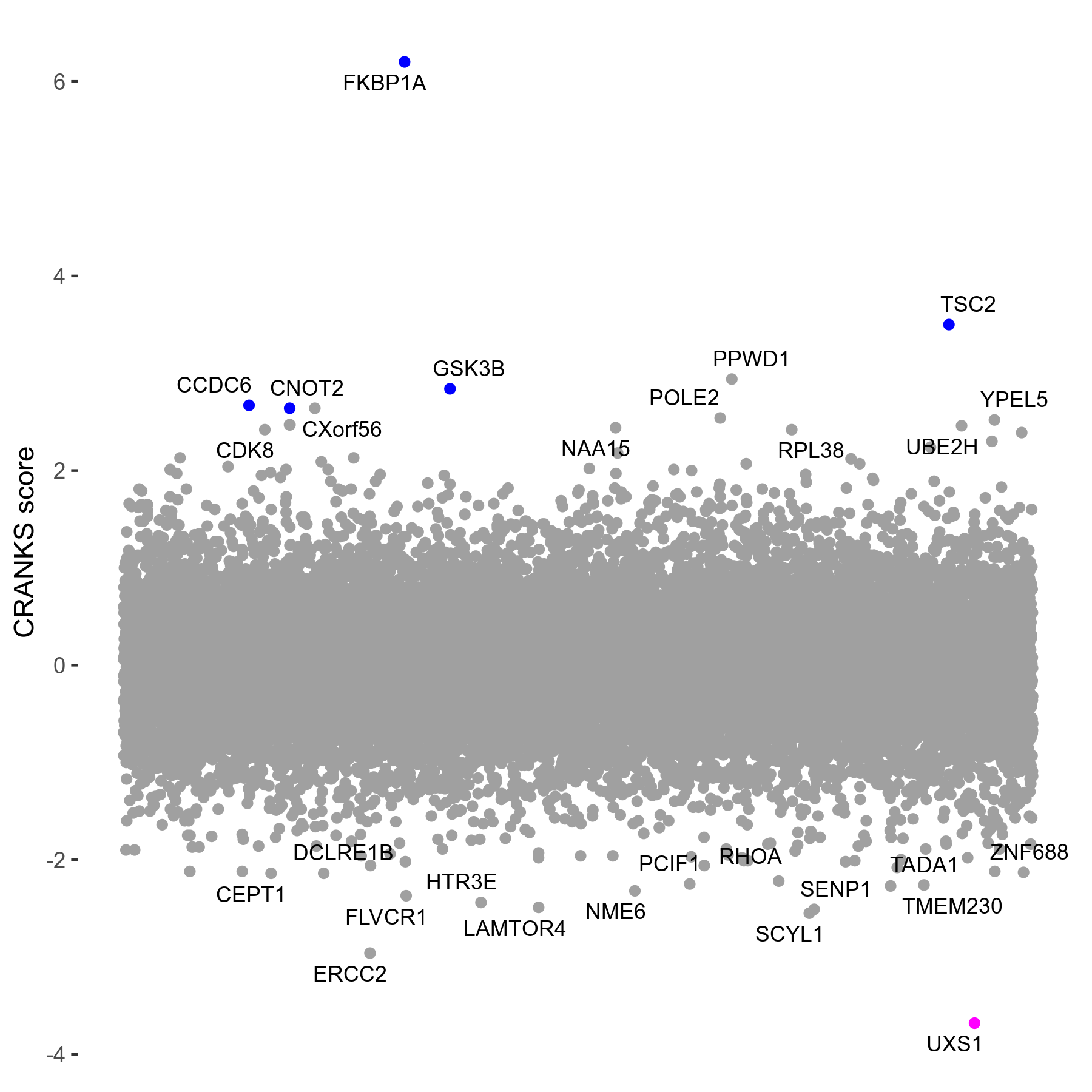
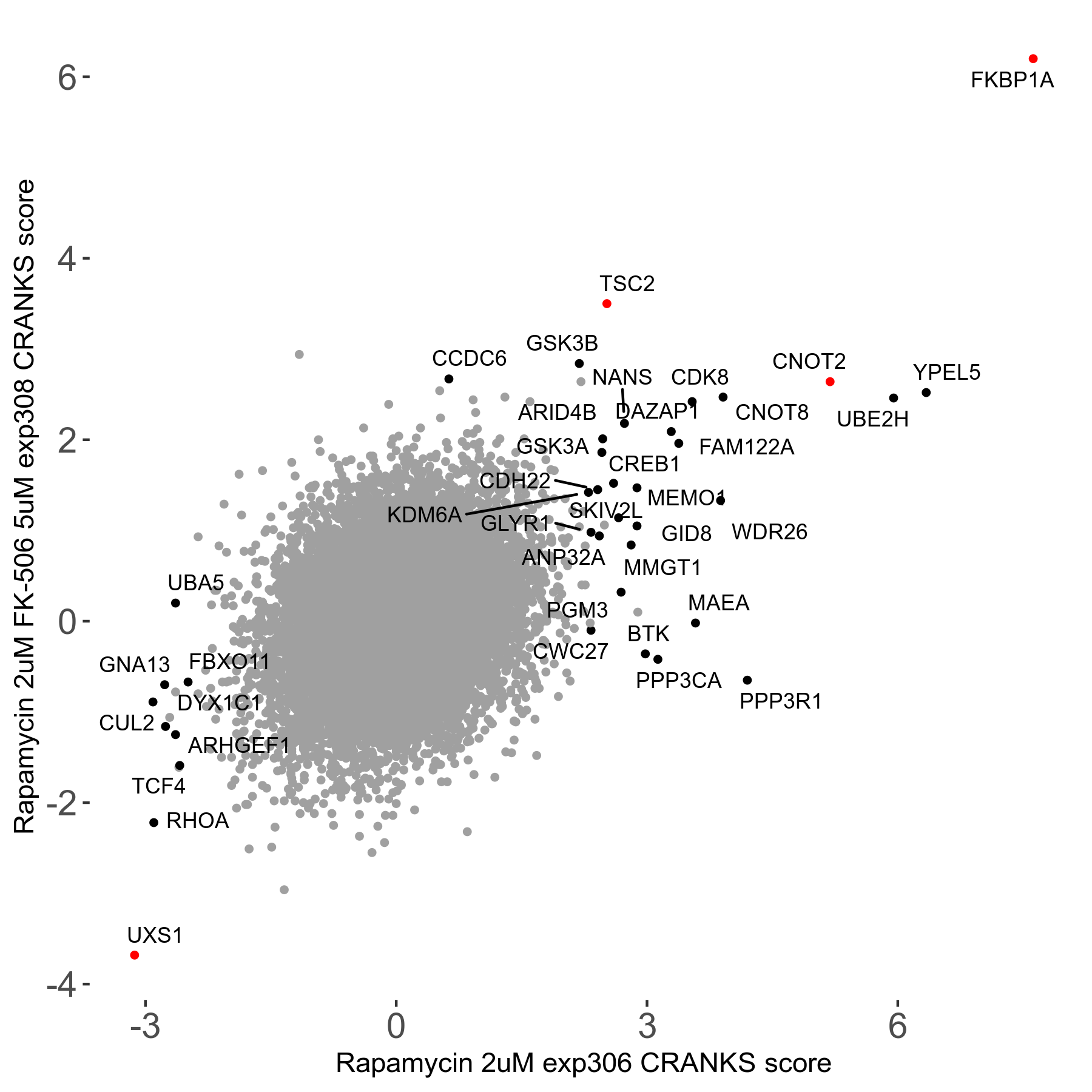
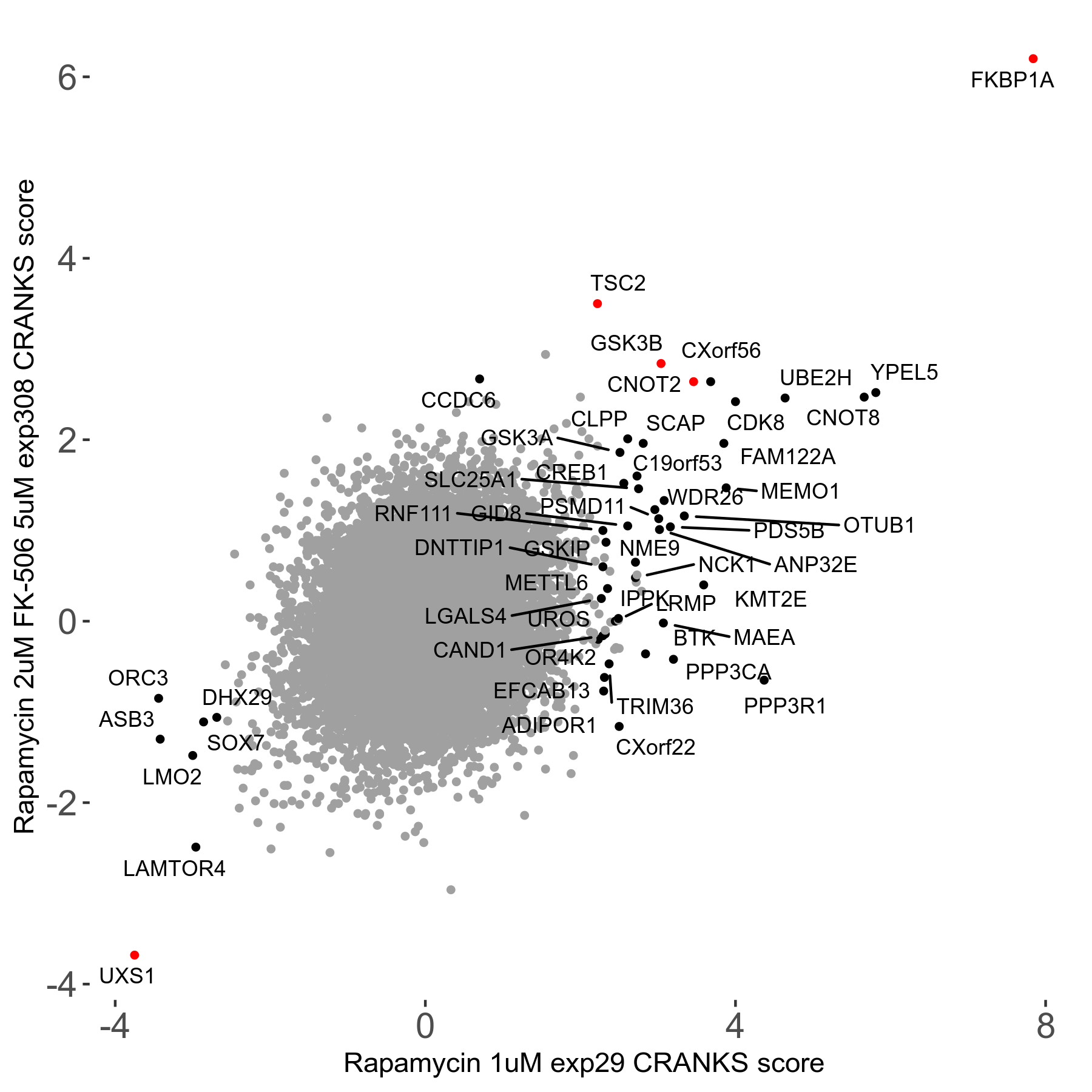
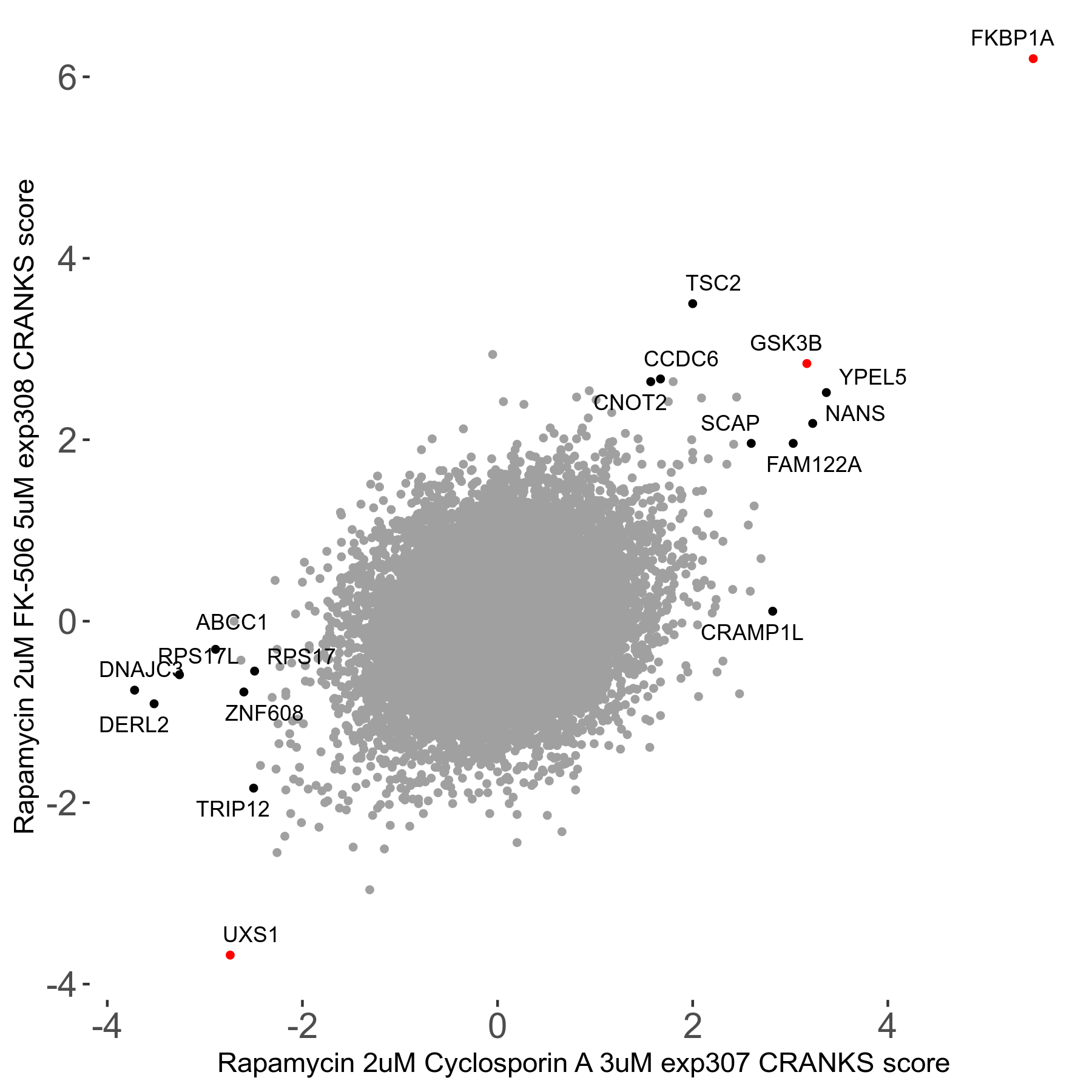
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 1/5 | Scores |