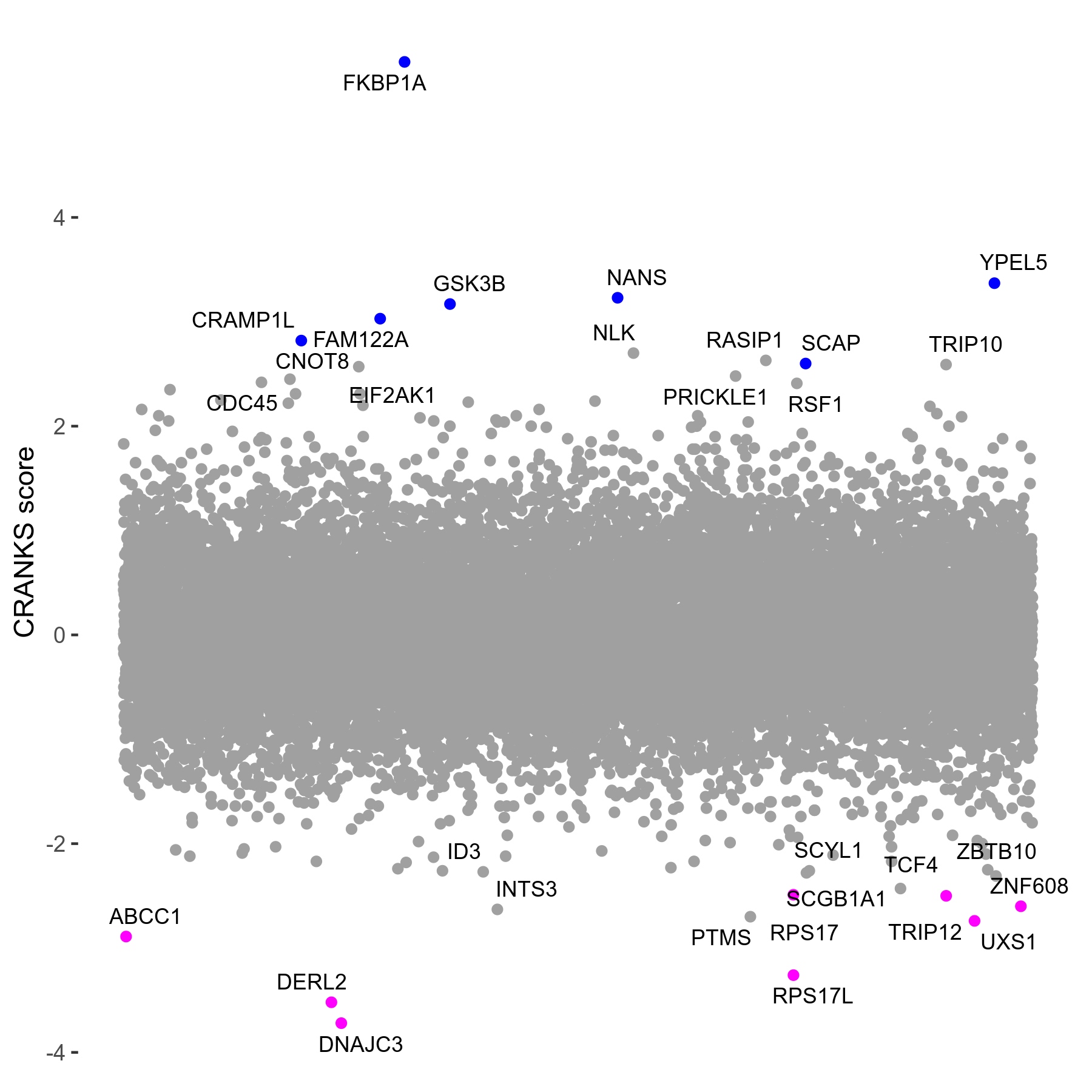
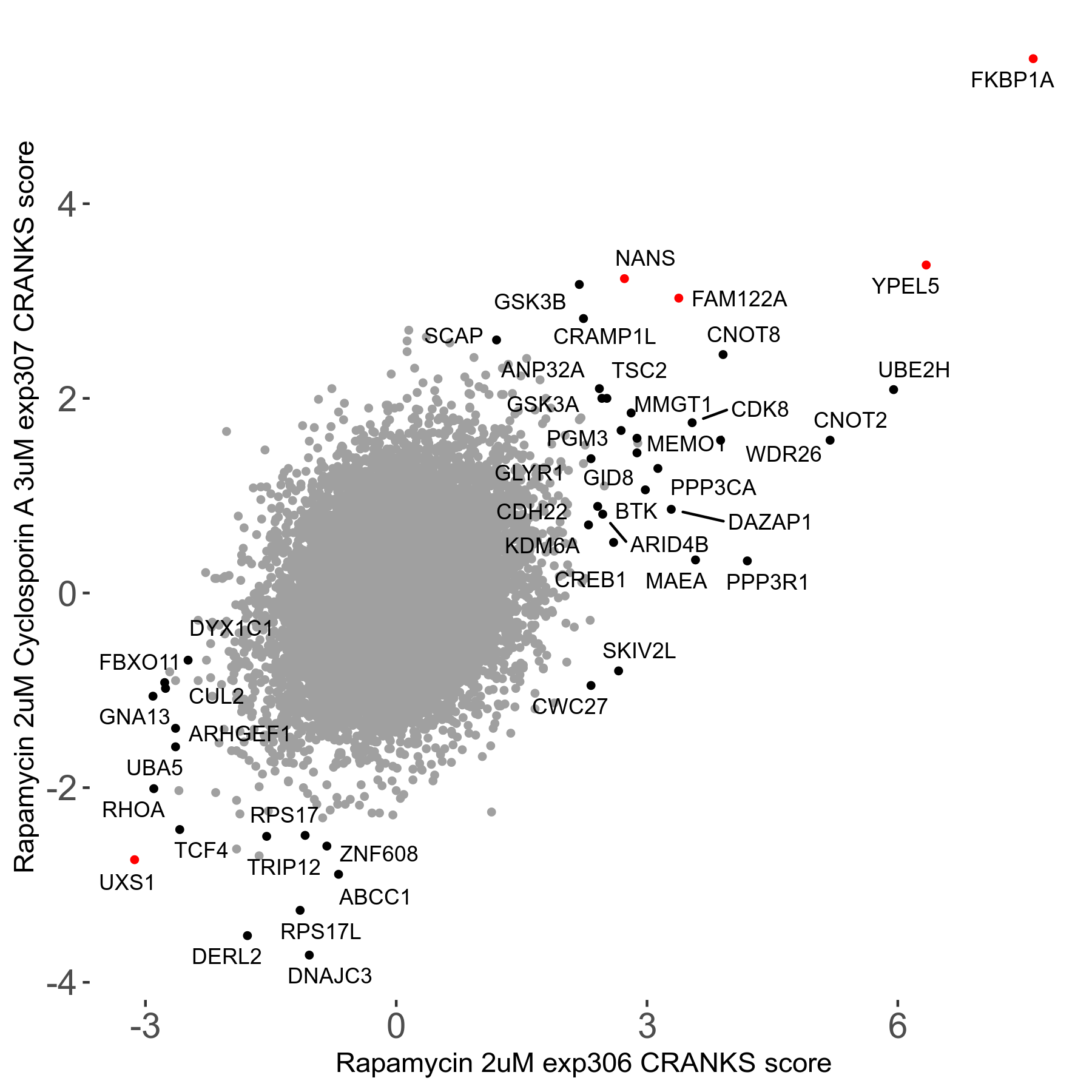
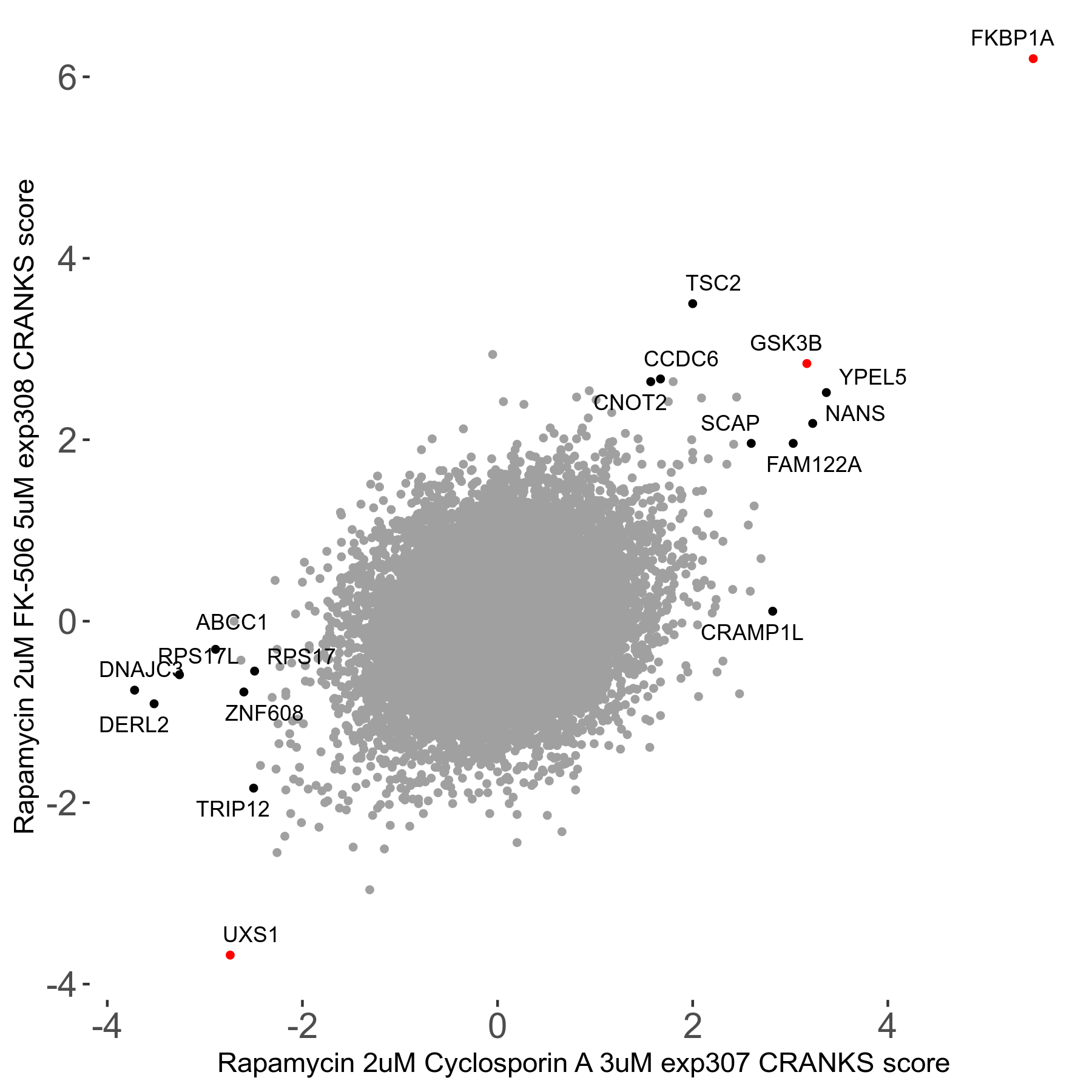
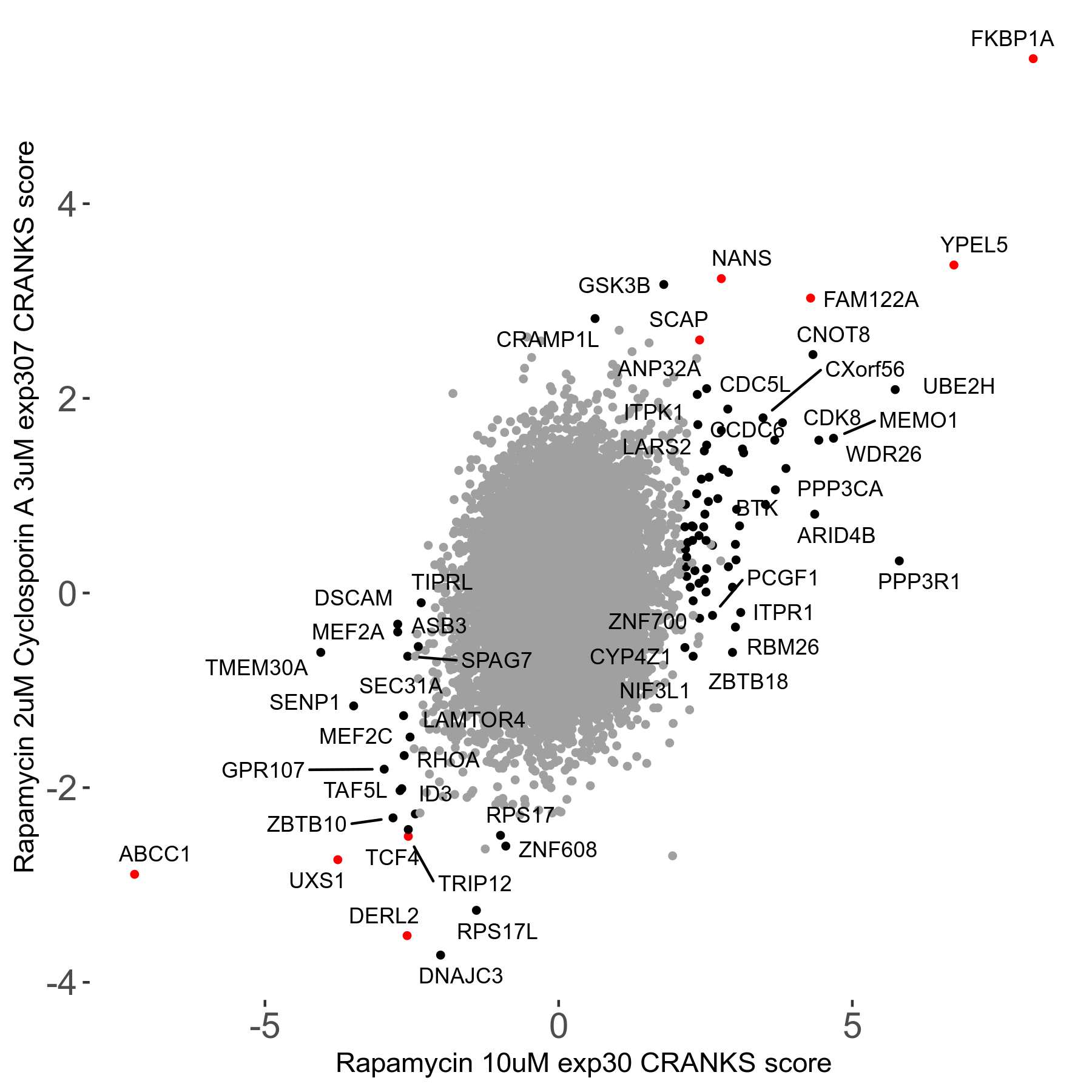
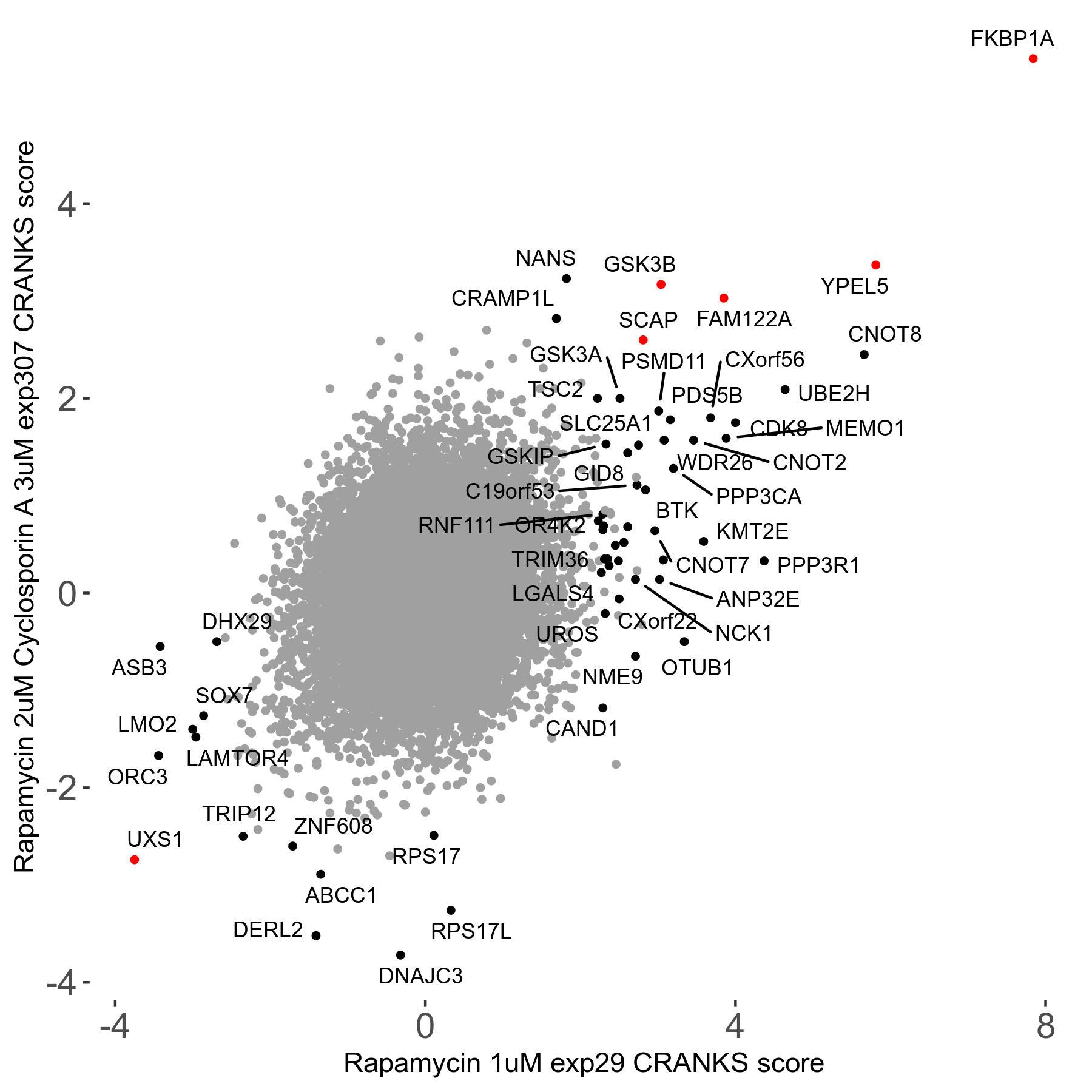
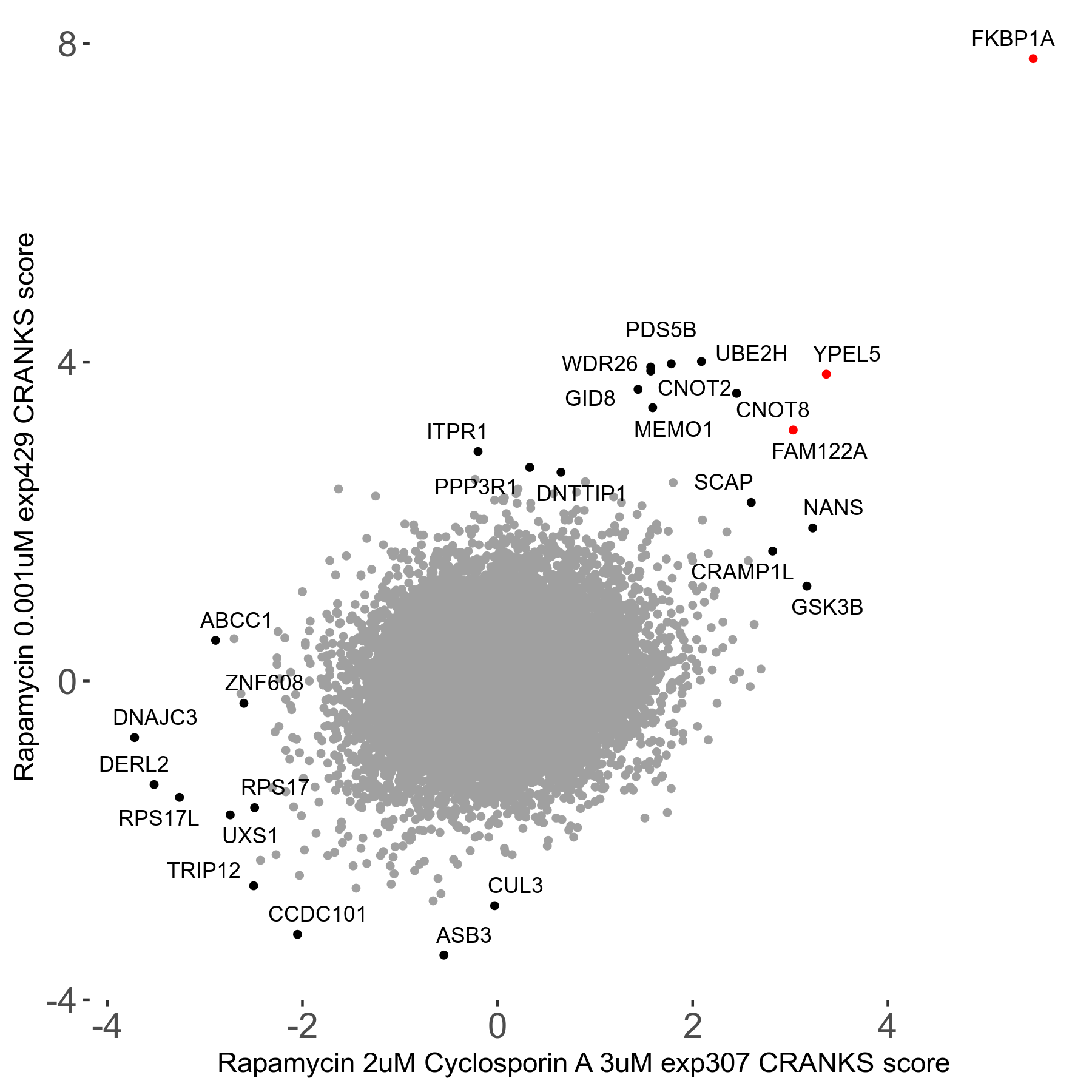
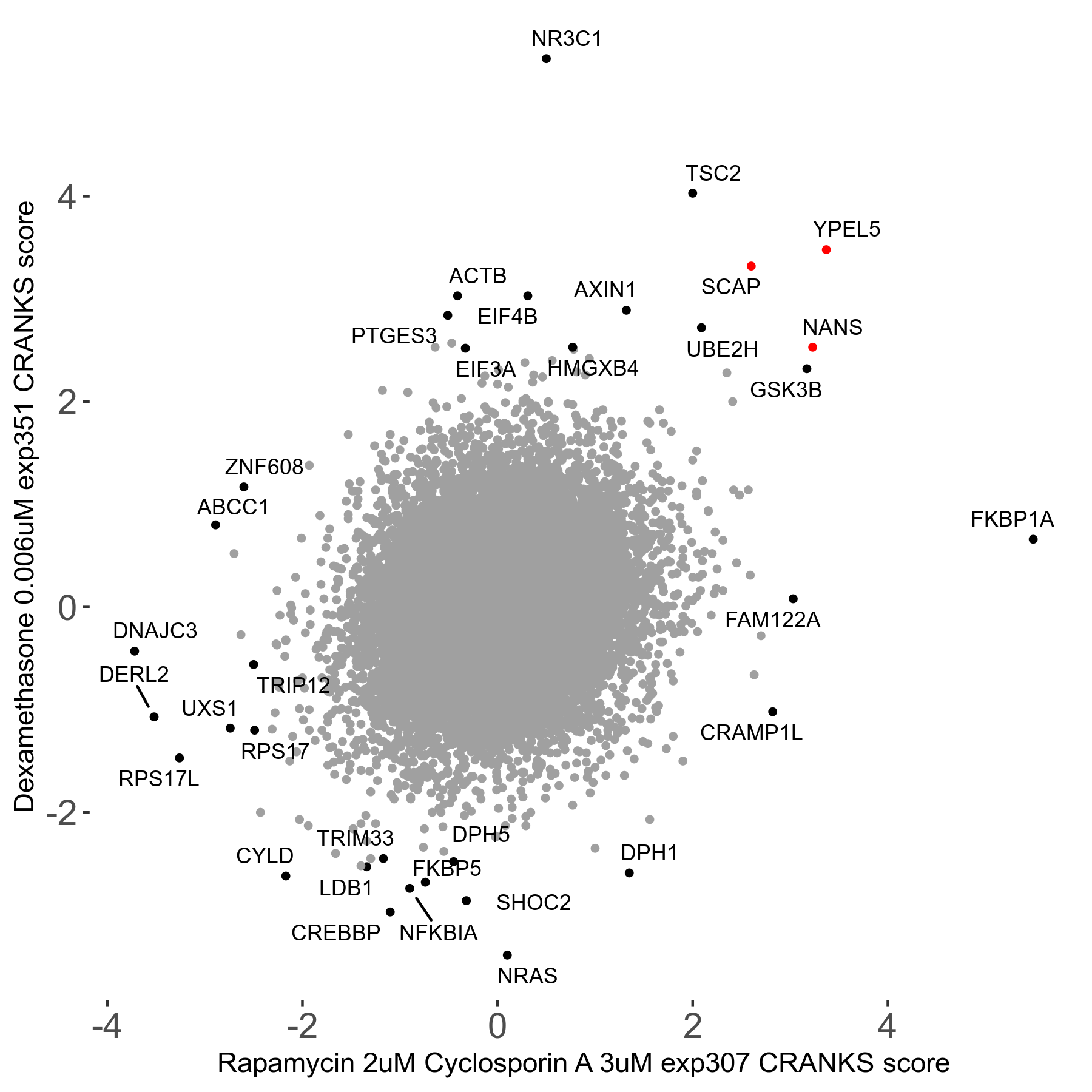
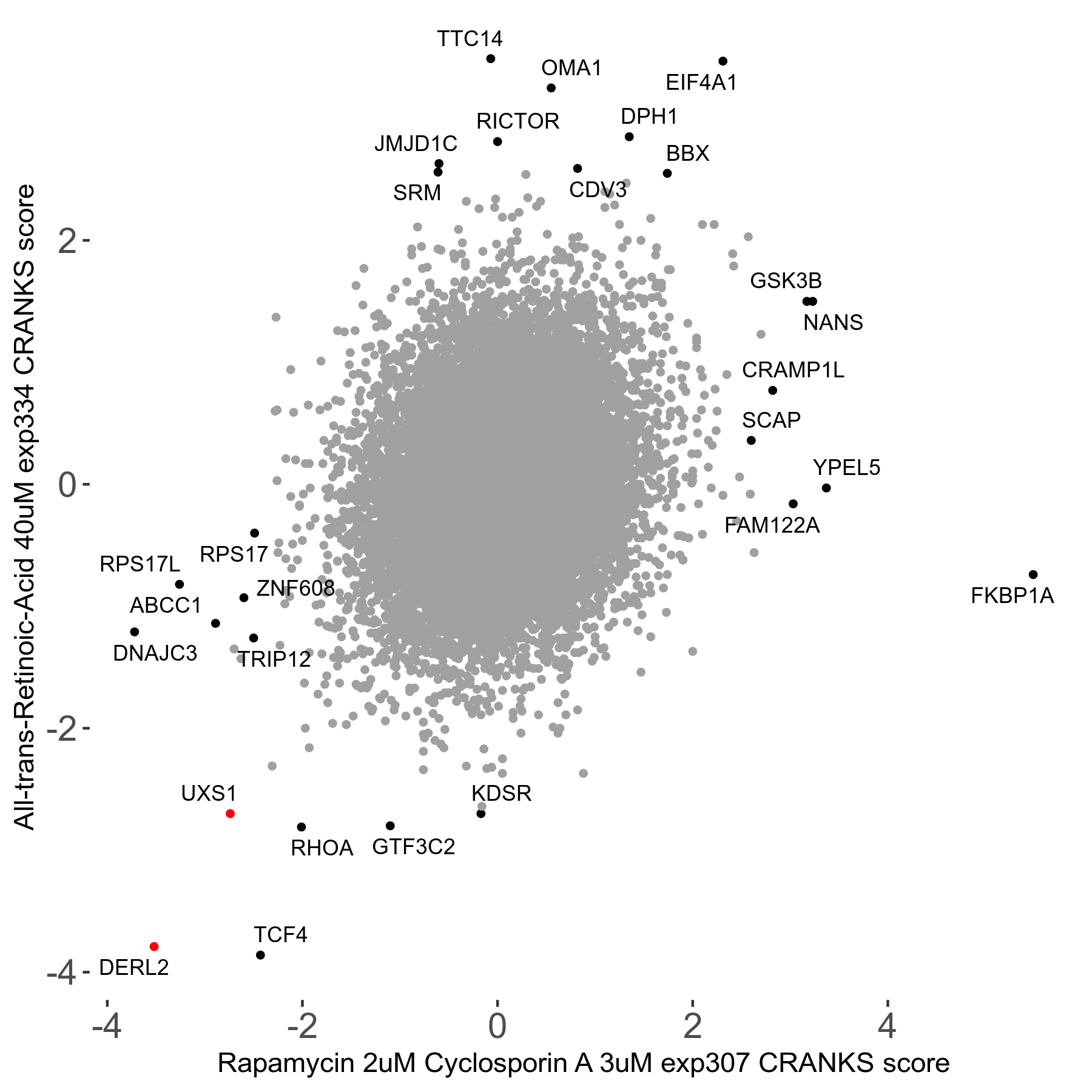
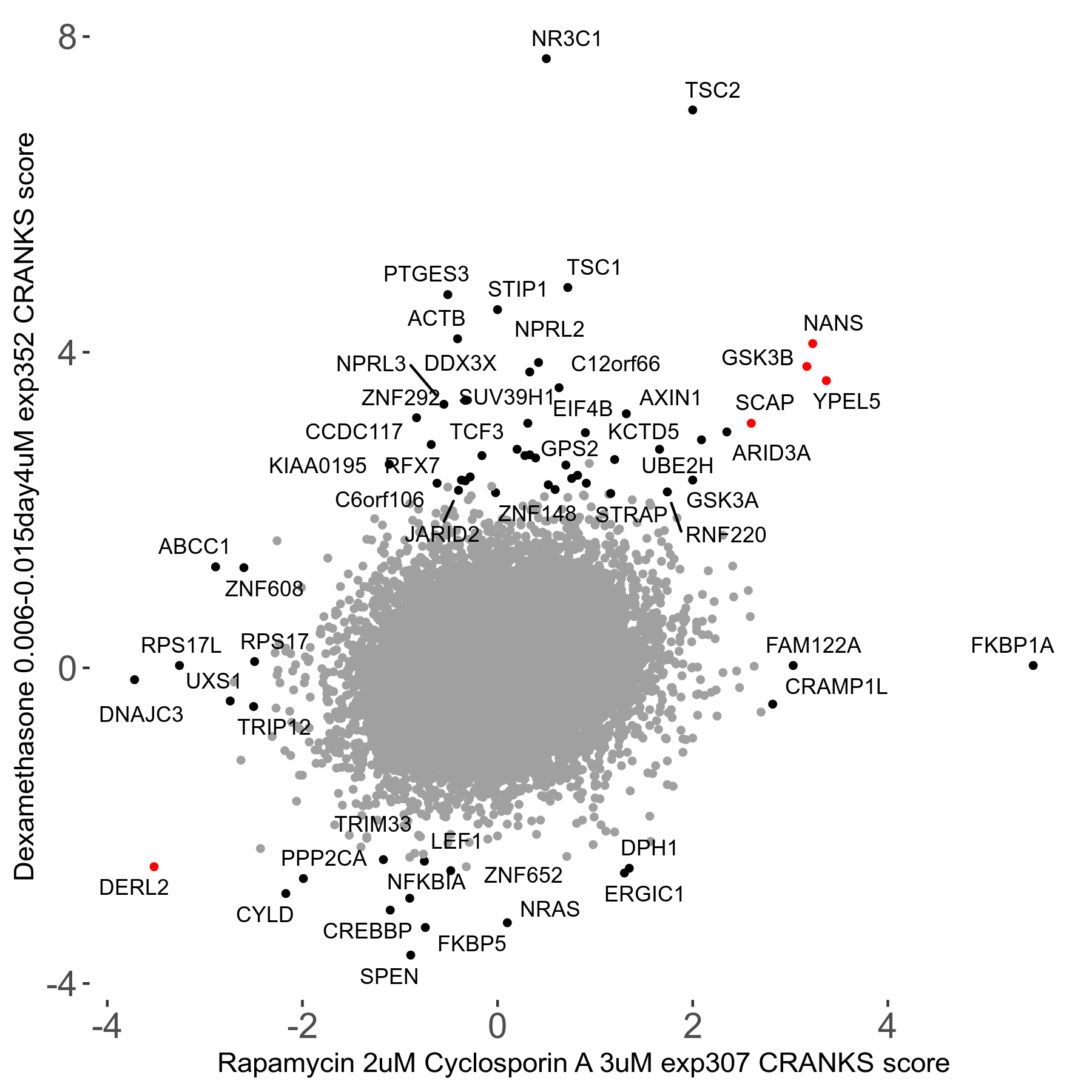
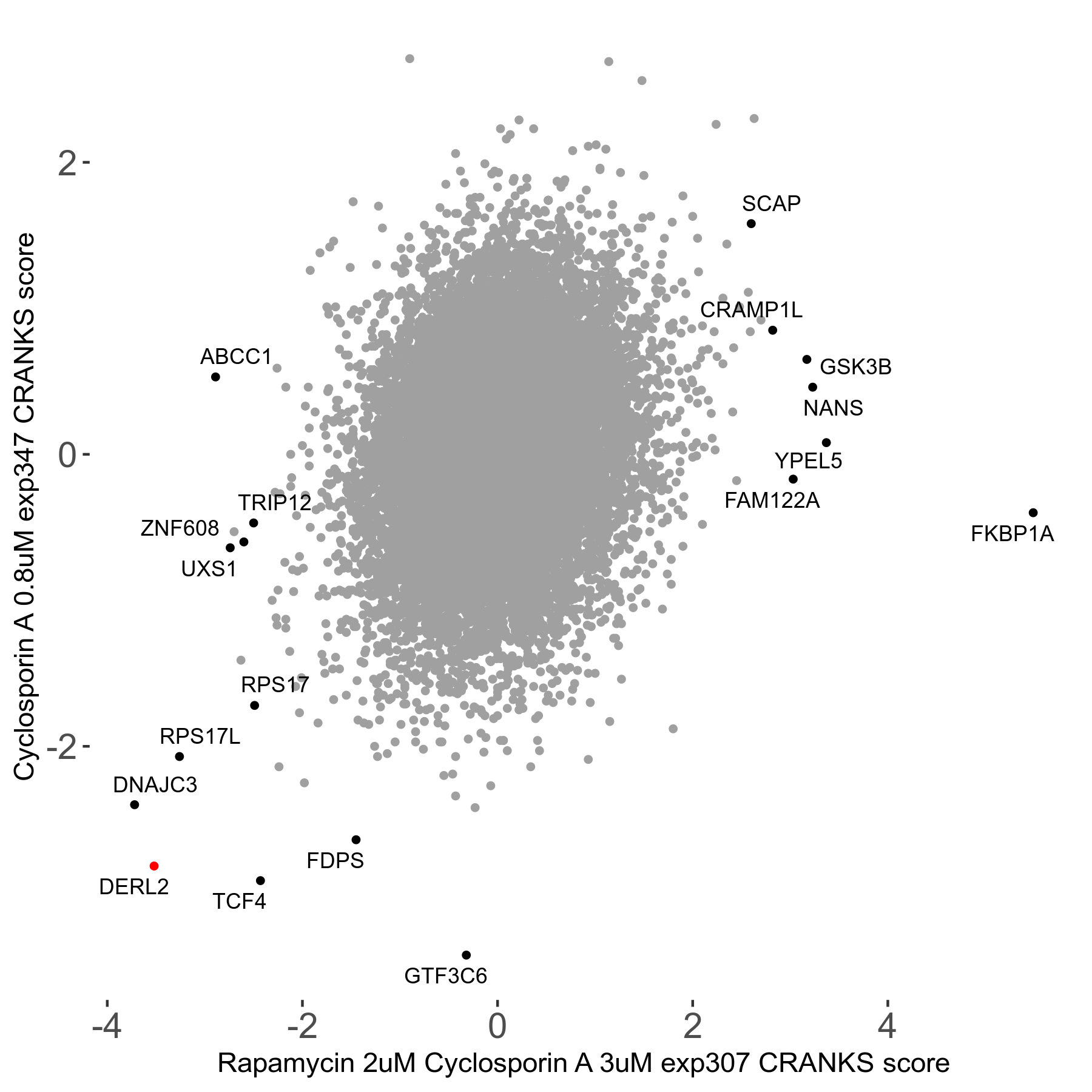
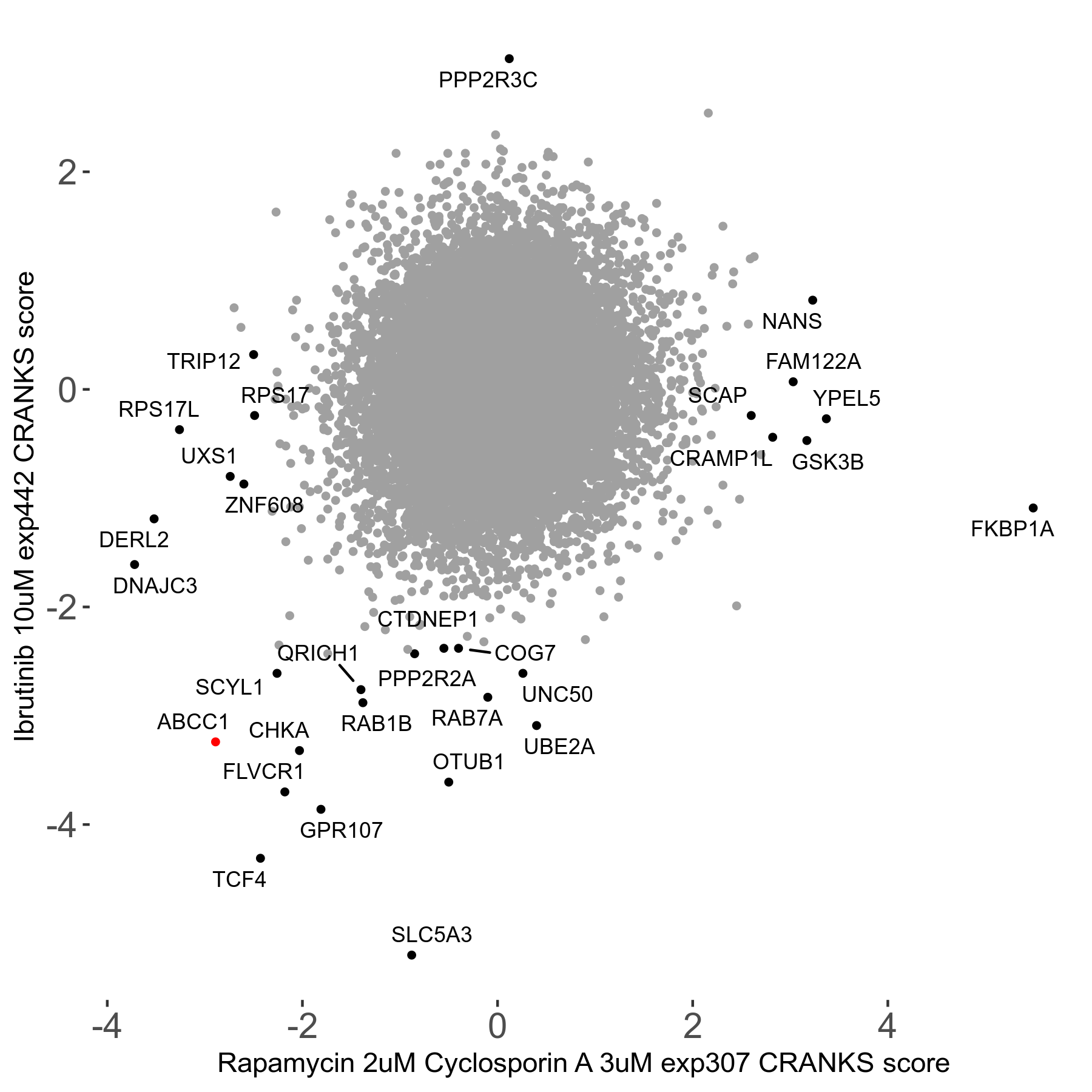
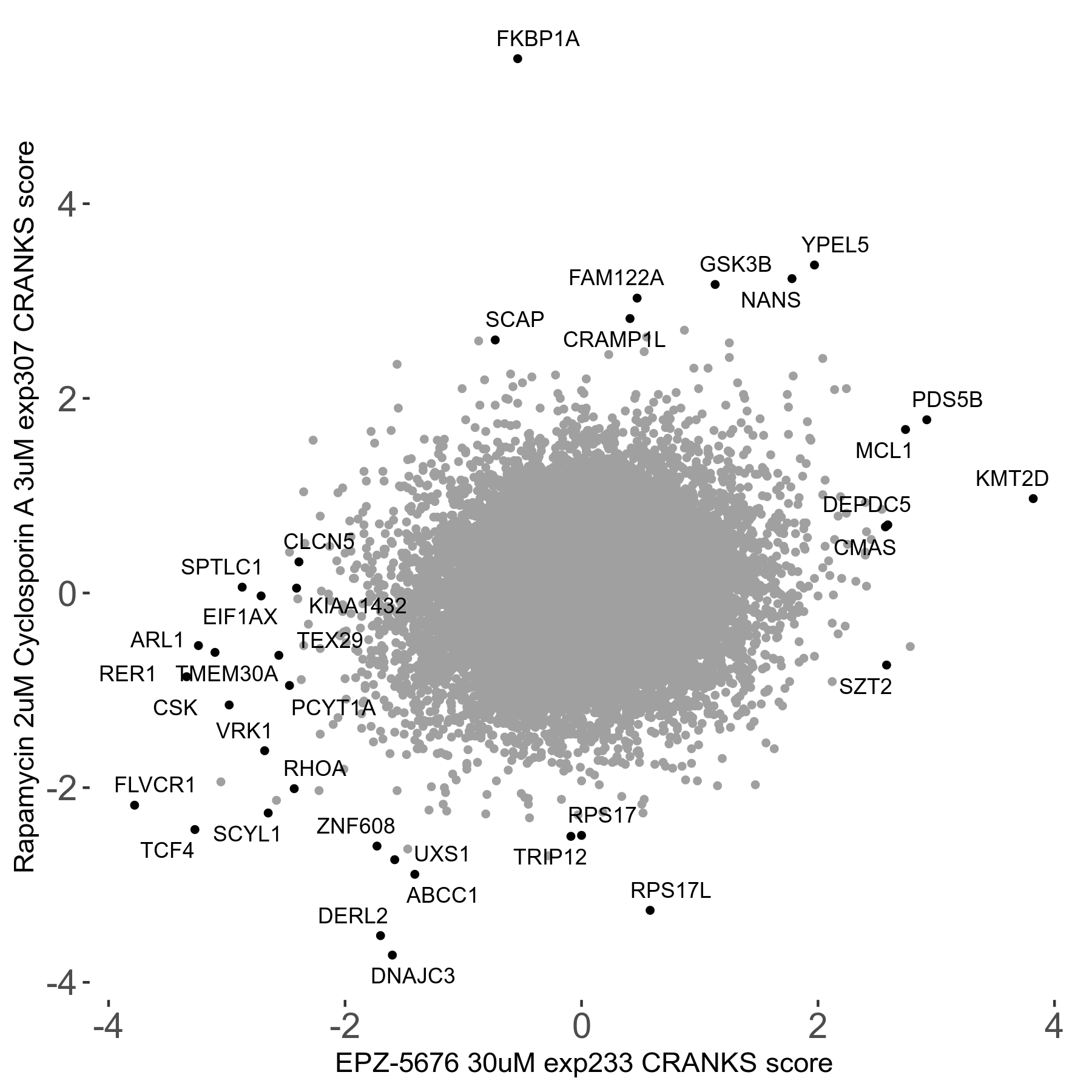
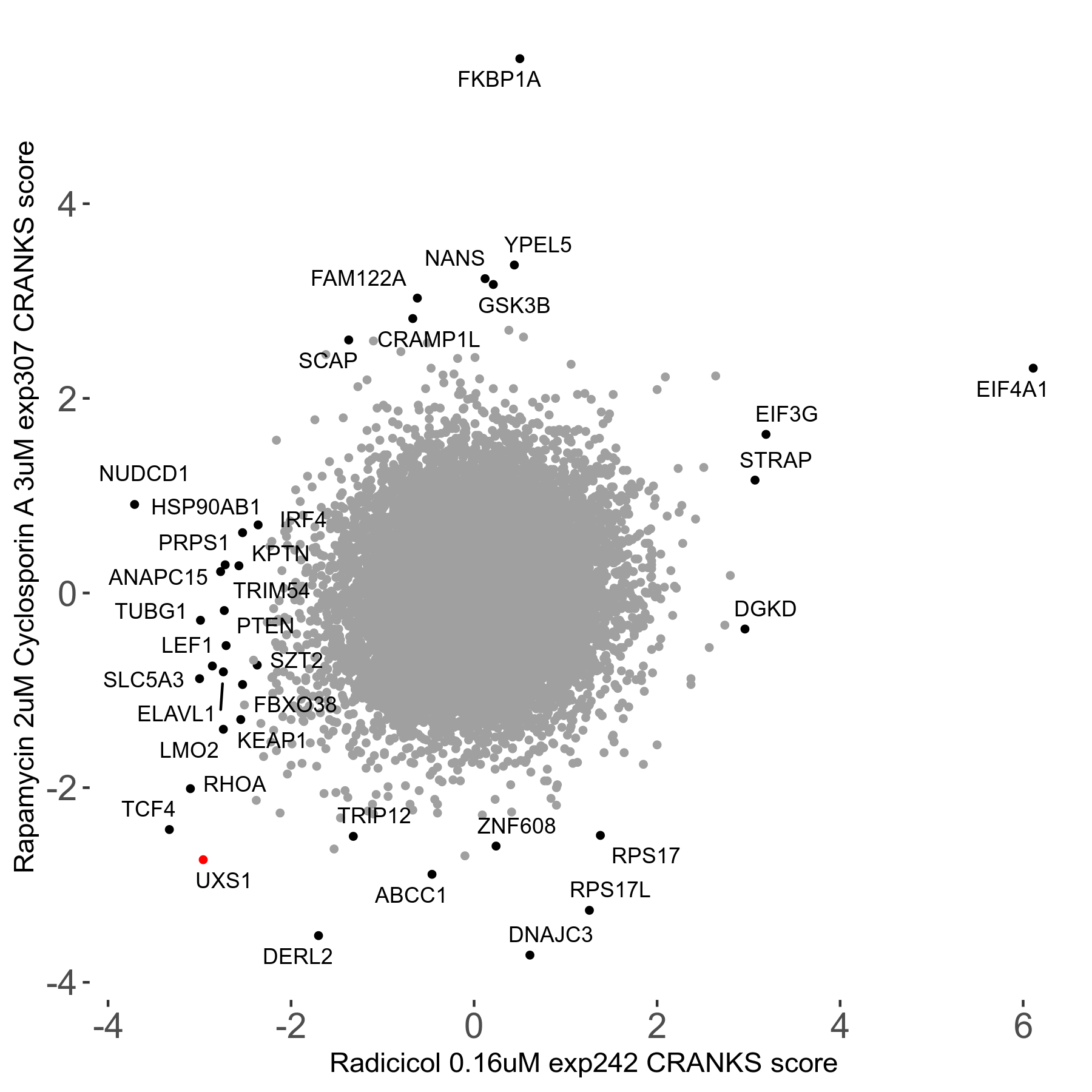
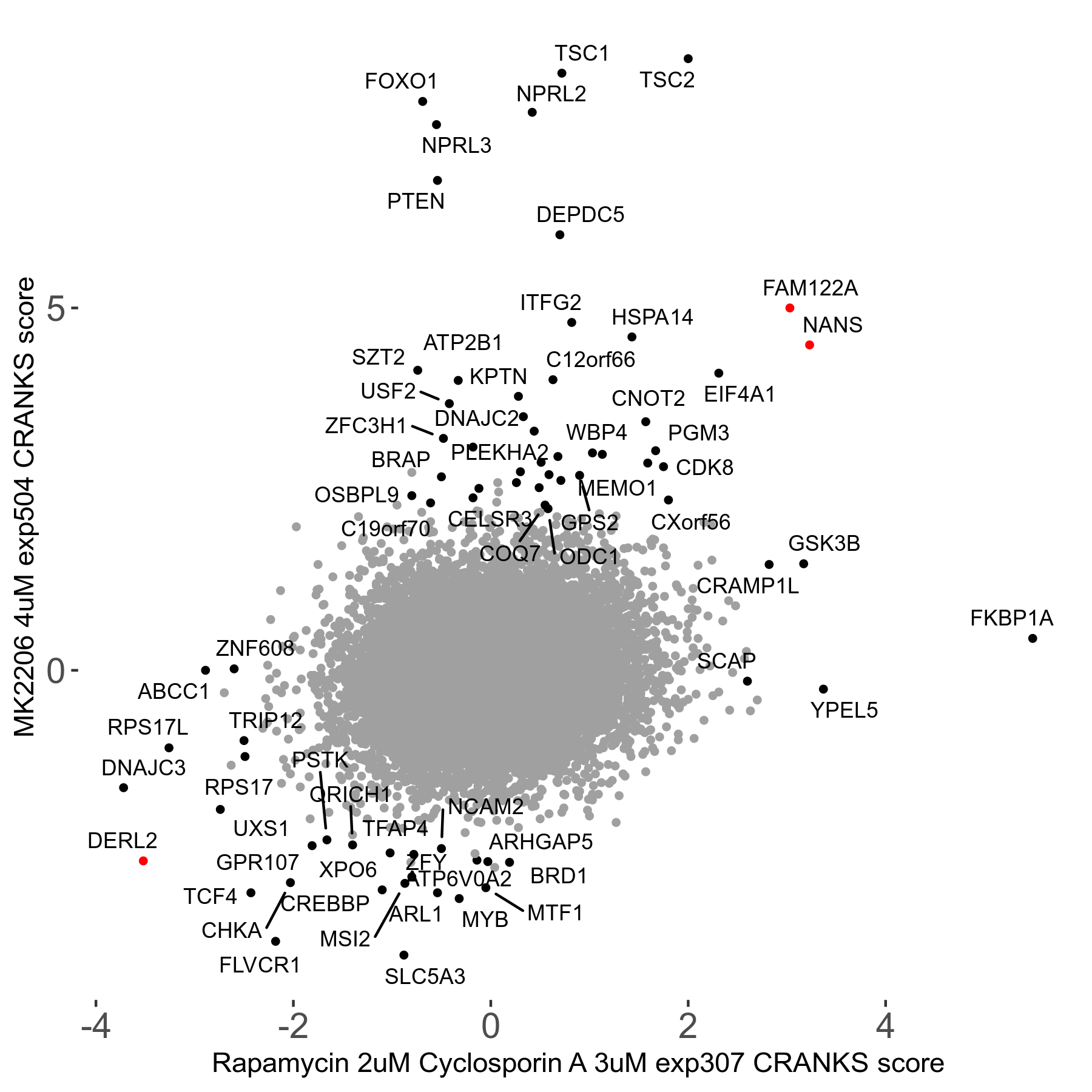
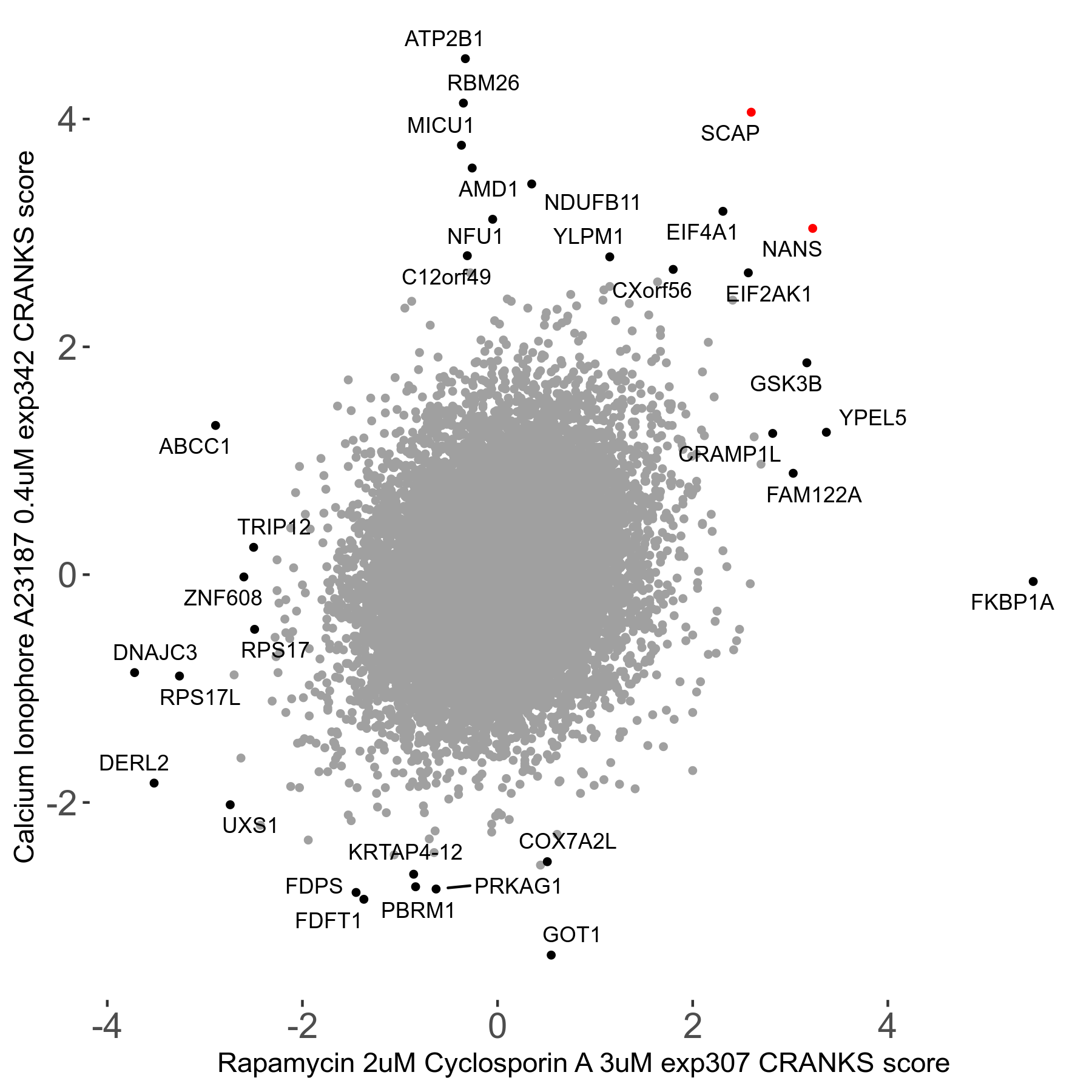
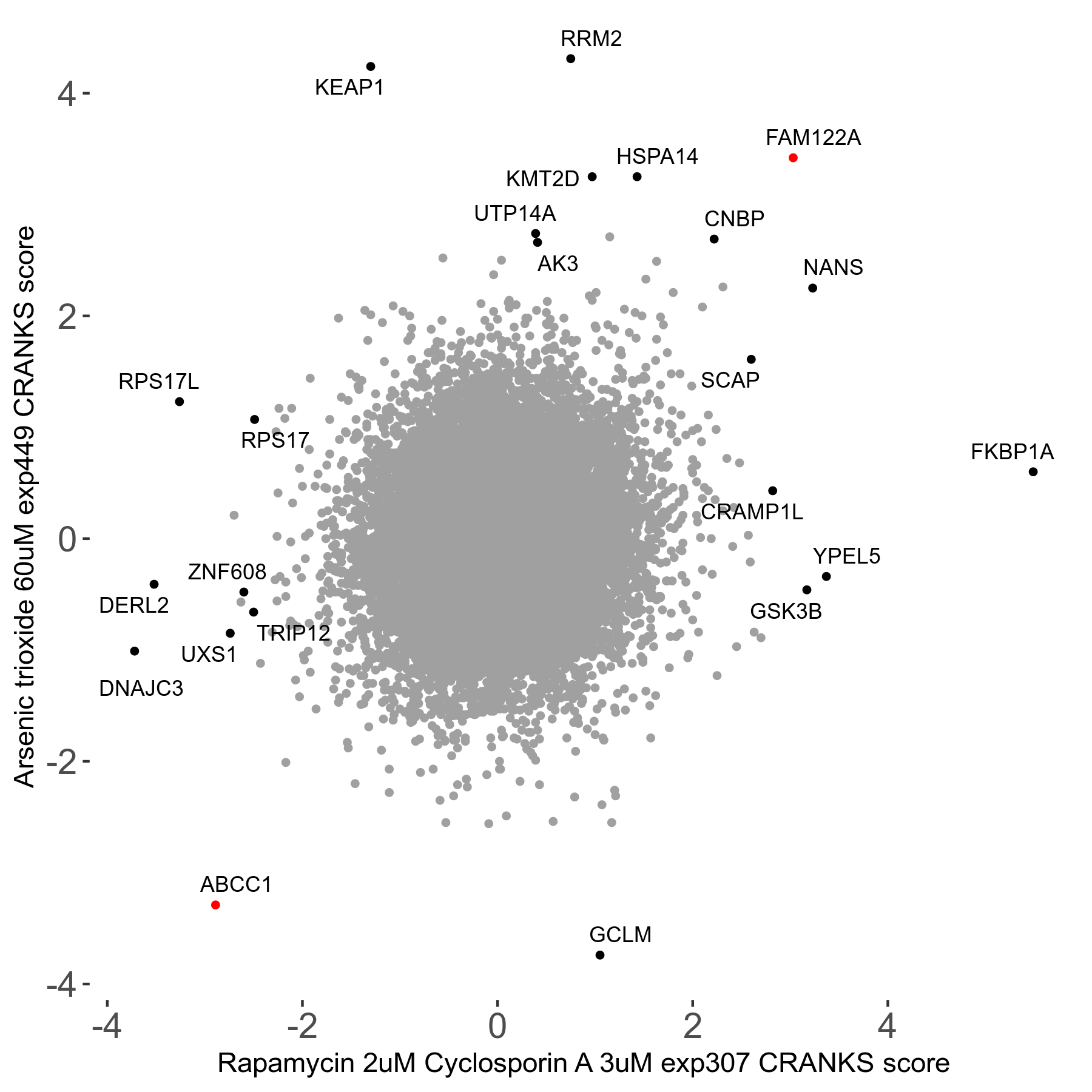
Rapamycin 2μM plus Cyclosporin-A 3μM R07 exp307
Mechanism of Action
Allosteric mTORC1 inhibitor, forms ternary complex with FKBP12 and mTOR, attenuates protein synthesis and metabolism
- Class / Subclass 1: Signal Transduction / Kinase Inhibitor
- Class / Subclass 2: Proteostasis / mTOR inhibitor
Technical Notes
Rapamycin
Compound References
- PubChem Name: Sirolimus
- Synonyms: Sirolimus; AY 22989
- CAS #: 53123-88-9
- PubChem CID: 5284616
- IUPAC: (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18-dihydroxy-12-[(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone
- INCHI Name: InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1
- INCHI Key: QFJCIRLUMZQUOT-HPLJOQBZSA-N
- Molecular Weight: 914.2
- Canonical SMILES: CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)O)C)C)O)OC)C)C)C)OC
- Isomeric SMILES: C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)O)C)/C)O)OC)C)C)/C)OC
- Molecular Formula: C51H79NO13
Compound Supplier
- Supplier Name: LC Laboratories
- Catalog #: R-5000
- Lot #: ASW-130
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C51H79NO13 936.54436; found 936.54366
Cyclosporin A
Compound References
- PubChem Name: Cyclosporin A
- Synonyms: Cyclosporine; Ciclosporin
- CAS #: 59865-13-3
- PubChem CID: 5284373
- IUPAC: (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(E,1R,2R)-1-hydroxy-2-methylhex-4-enyl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone
- INCHI Name: InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1
- INCHI Key: PMATZTZNYRCHOR-CGLBZJNRSA-N
- Molecular Weight: 1202.6
- Canonical SMILES: CCC1C(=O)N(CC(=O)N(C(C(=O)NC(C(=O)N(C(C(=O)NC(C(=O)NC(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N1)C(C(C)CC=CC)O)C)C(C)C)C)CC(C)C)C)CC(C)C)C)C)C)CC(C)C)C)C(C)C)CC(C)C)C)C
- Isomeric SMILES: CC[C@H]1C(=O)N(CC(=O)N([C@H](C(=O)N[C@H](C(=O)N([C@H](C(=O)N[C@H](C(=O)N[C@@H](C(=O)N([C@H](C(=O)N([C@H](C(=O)N([C@H](C(=O)N([C@H](C(=O)N1)[C@@H]([C@H](C)C/C=C/C)O)C)C(C)C)C)CC(C)C)C)CC(C)C)C)C)C)CC(C)C)C)C(C)C)CC(C)C)C)C
- Molecular Formula: C62H111N11O12
Compound Supplier
- Supplier Name: Sigma-Aldrich
- Catalog #: 30024
- Lot #: 142714V
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C62H111N11O12 1202.84864; found 1202.84948
Rapamycin+Cyclosporin A
Dose Response Curve
- Platform ID: CyclosporinA_3uM_Rapamycin
- Min: 1.4508; Max: 90.9009

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 3.3691 |
| IC30 | 4.5493 |
| IC40 | 5.8239 |
| IC50 | 7.3402 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
The dose response curve for Rapamycin is also available at:
The dose response curve for Cyclosporin A is also available at:
Screen Summary
- Round: 07
- Dose: 2µM + 3µM
- Days of incubation: 8
- Doublings: 5.0
- Numbers of reads: 17458875
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 8/7 | Scores |