Table of Contents
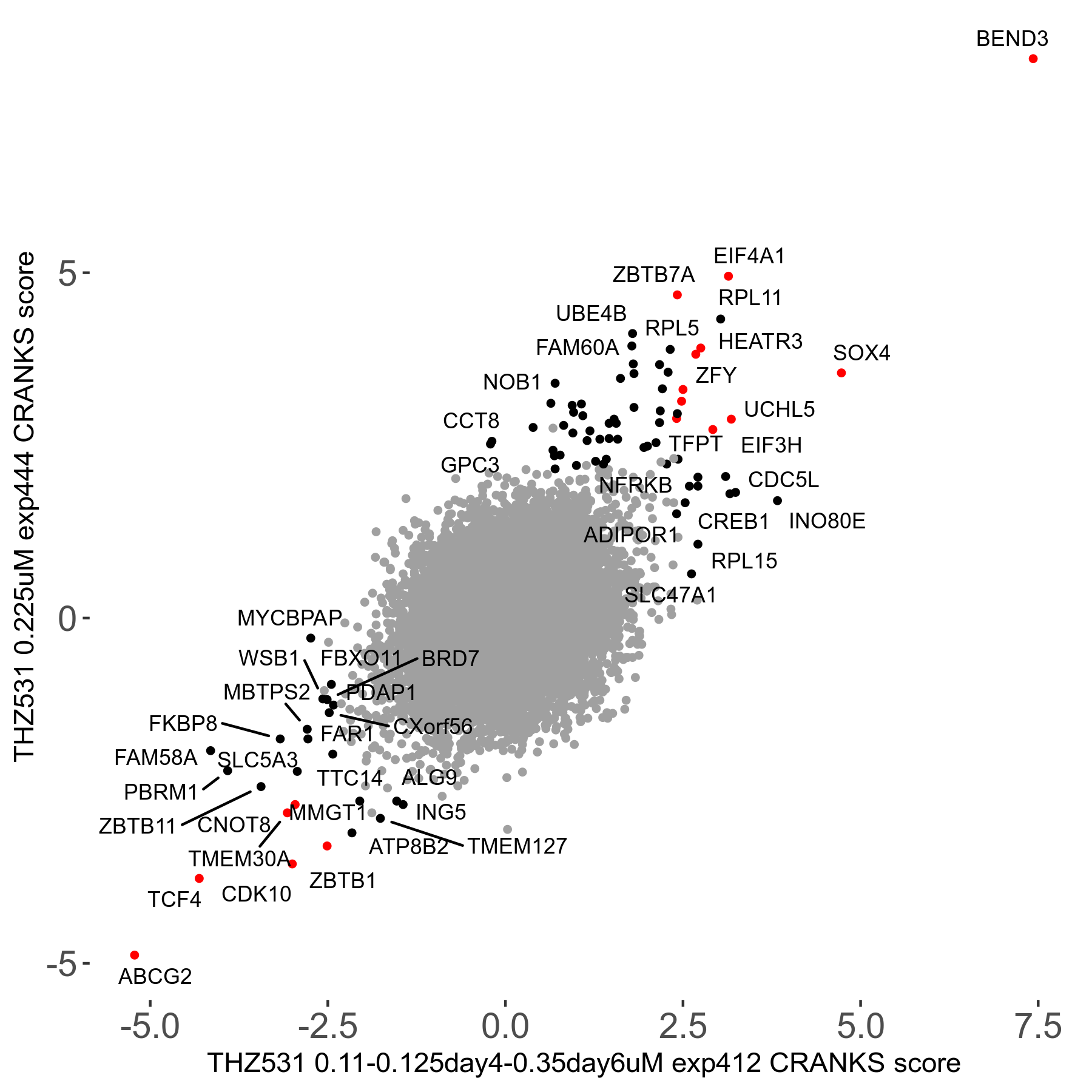
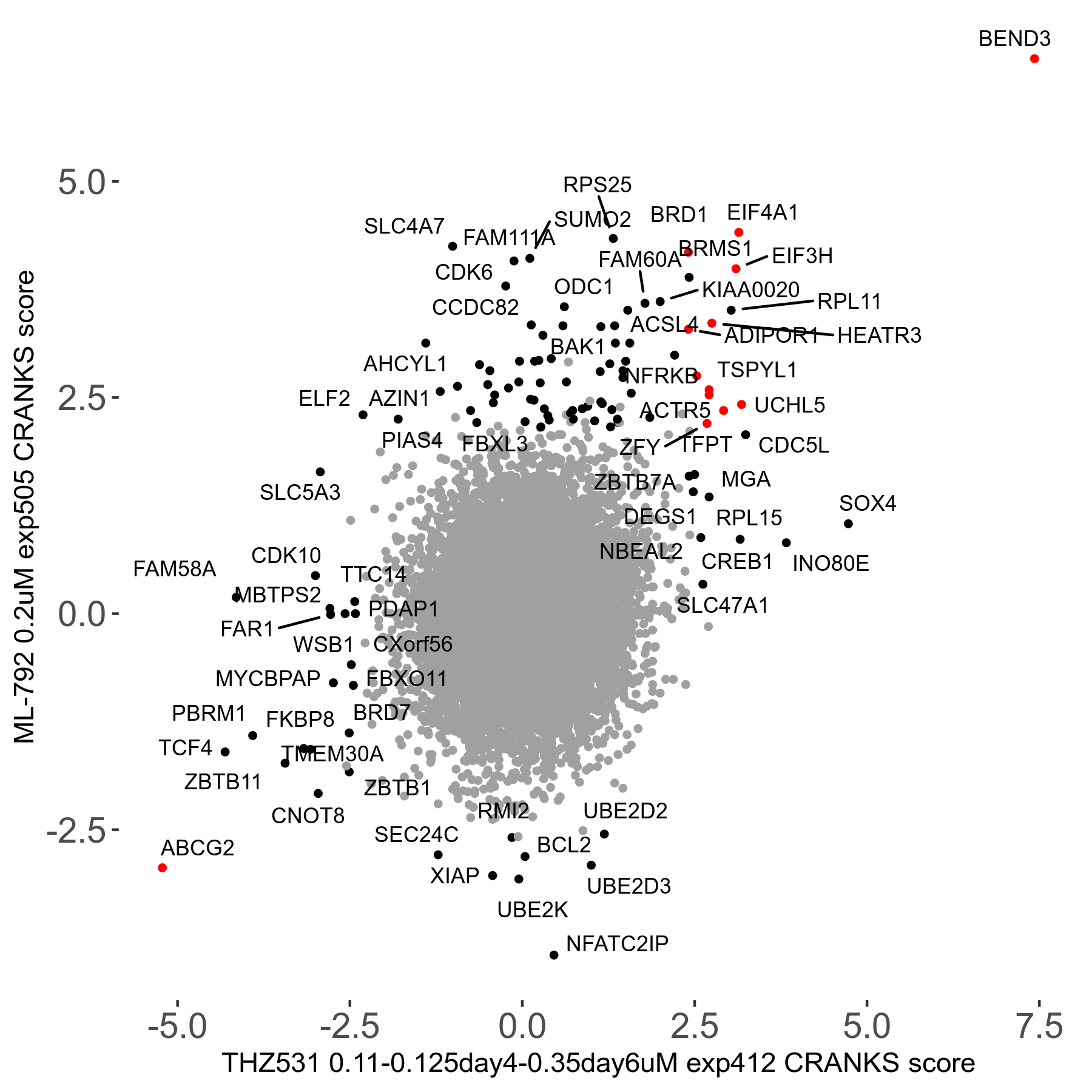
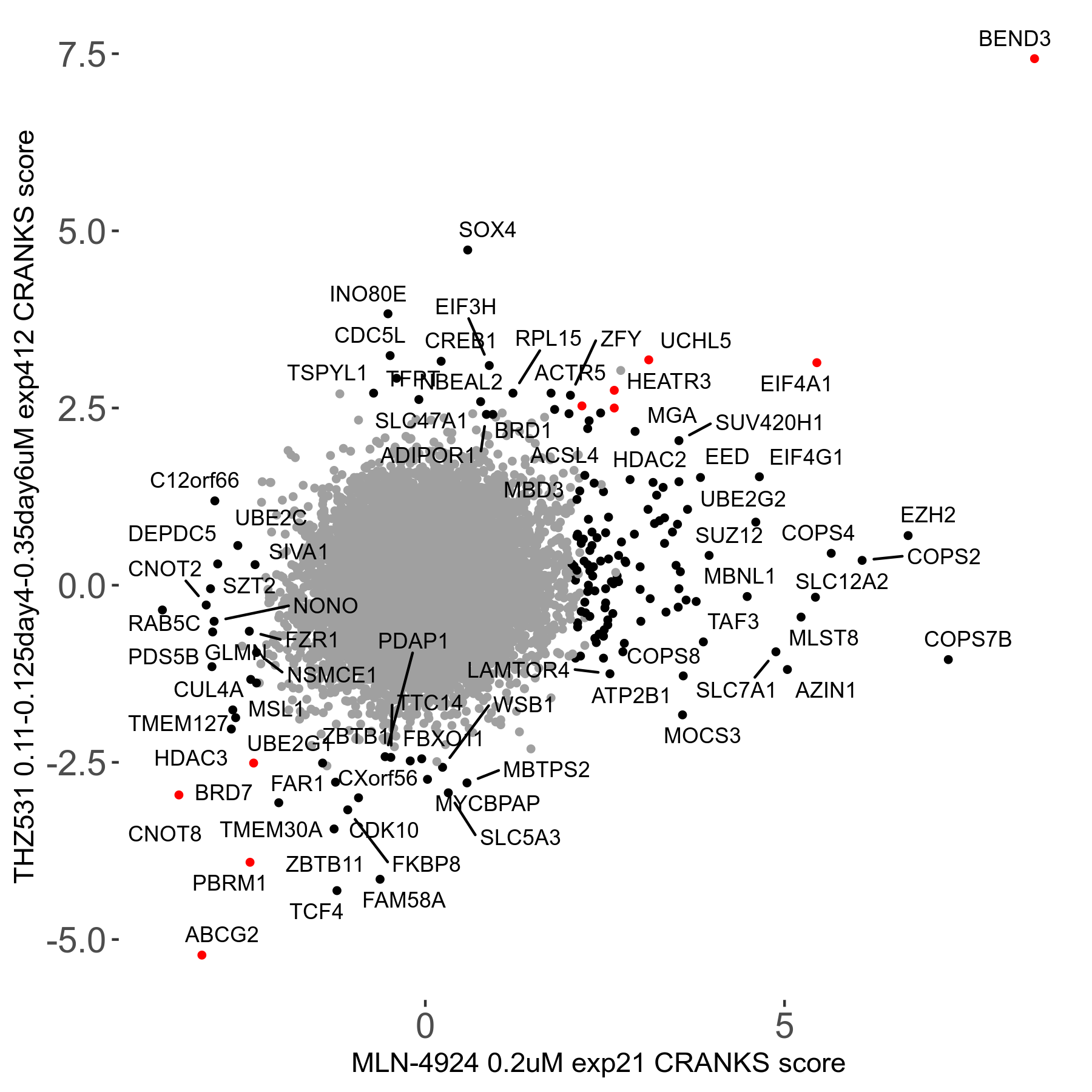
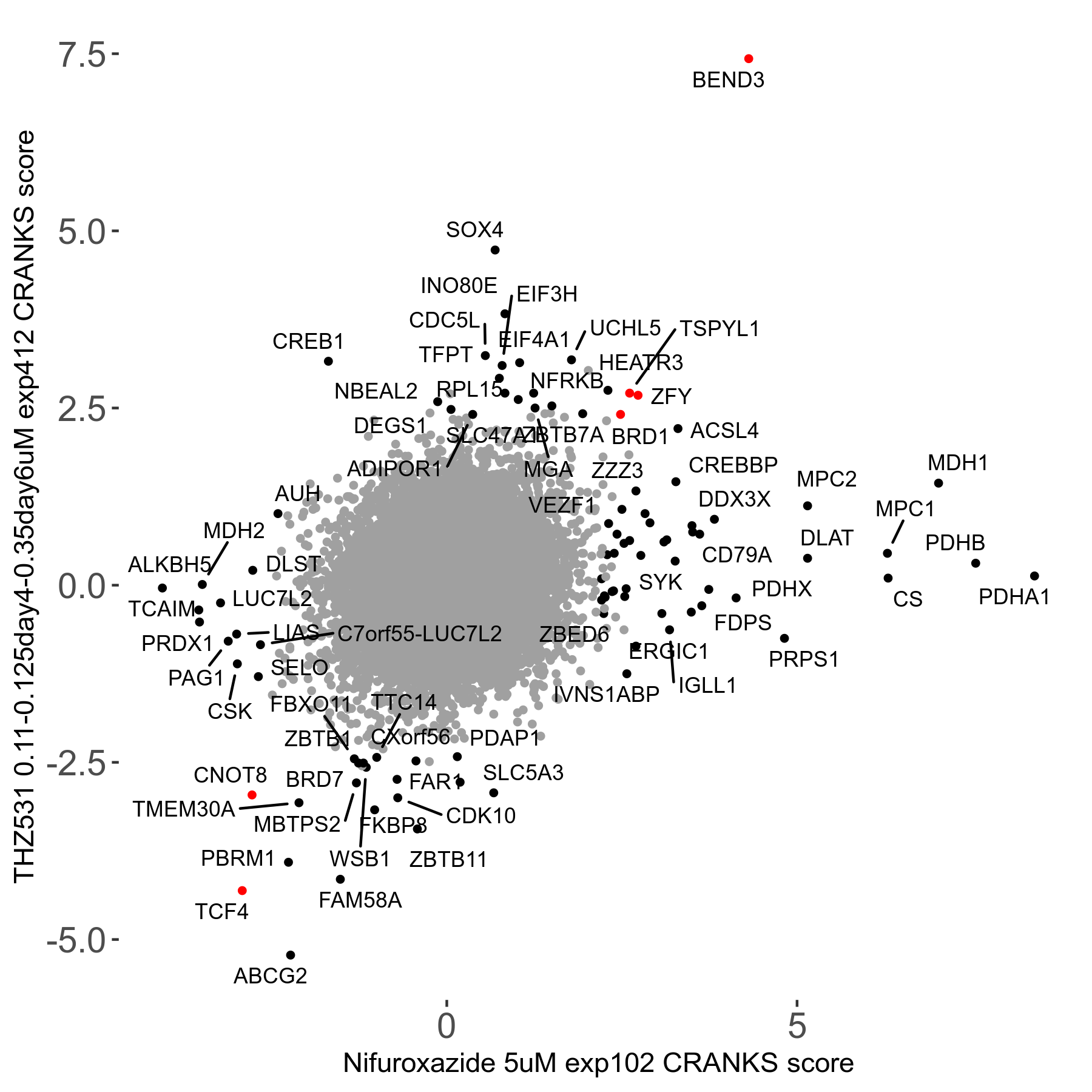
THZ531 0.11 to 0.125 to 0.35μM on day4 then day6 R07 exp412
Mechanism of Action
Inhibits CDK12 and CDK13, forms covalent adduct, more selective analog of THZ1, blocks phosphorylation of the C-terminal domain of RNA polymerase II
- Class / Subclass 1: Gene Regulation / Transcription Inhibitor
- Class / Subclass 2: Signal Transduction / Kinase Inhibitor
Technical Notes
Protein References
- PubChem Name: (E)-N-[4-[(3R)-3-[[5-Chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]piperidine-1-carbonyl]phenyl]-4-(dimethylamino)but-2-enamide
- Synonyms: N/A
- CAS #: 1702809-17-3
- PubChem CID: 118025540
- IUPAC: (E)-N-[4-[(3R)-3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]piperidine-1-carbonyl]phenyl]-4-(dimethylamino)but-2-enamide
- INCHI Name: InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1
- INCHI Key: RUBYHLPRZRMTJO-MOVYNIQHSA-N
- Molecular Weight: 558.1
- Canonical SMILES: CN(C)CC=CC(=O)NC1=CC=C(C=C1)C(=O)N2CCCC(C2)NC3=NC=C(C(=N3)C4=CNC5=CC=CC=C54)Cl
- Isomeric SMILES: CN(C)C/C=C/C(=O)NC1=CC=C(C=C1)C(=O)N2CCC[C@H](C2)NC3=NC=C(C(=N3)C4=CNC5=CC=CC=C54)Cl
- Molecular Formula: C30H32ClN7O2
Protein Supplier
- Supplier Name: Med Chem Express
- Catalog #: HY-103618
- Lot #: 28264
Protein Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C30H32ClN7O2 558.23788; found 558.23943
Dose Response Curve
- Platform ID: THZ531
- Min: -7.6335; Max: 95.3893

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | 0.0546 |
| IC30 | 0.0716 |
| IC40 | 0.0910 |
| IC50 | 0.1150 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 07
- Dose: 0.11-0.35µM
- Days of incubation: 8
- Doublings: 5.6
- Numbers of reads: 14317447
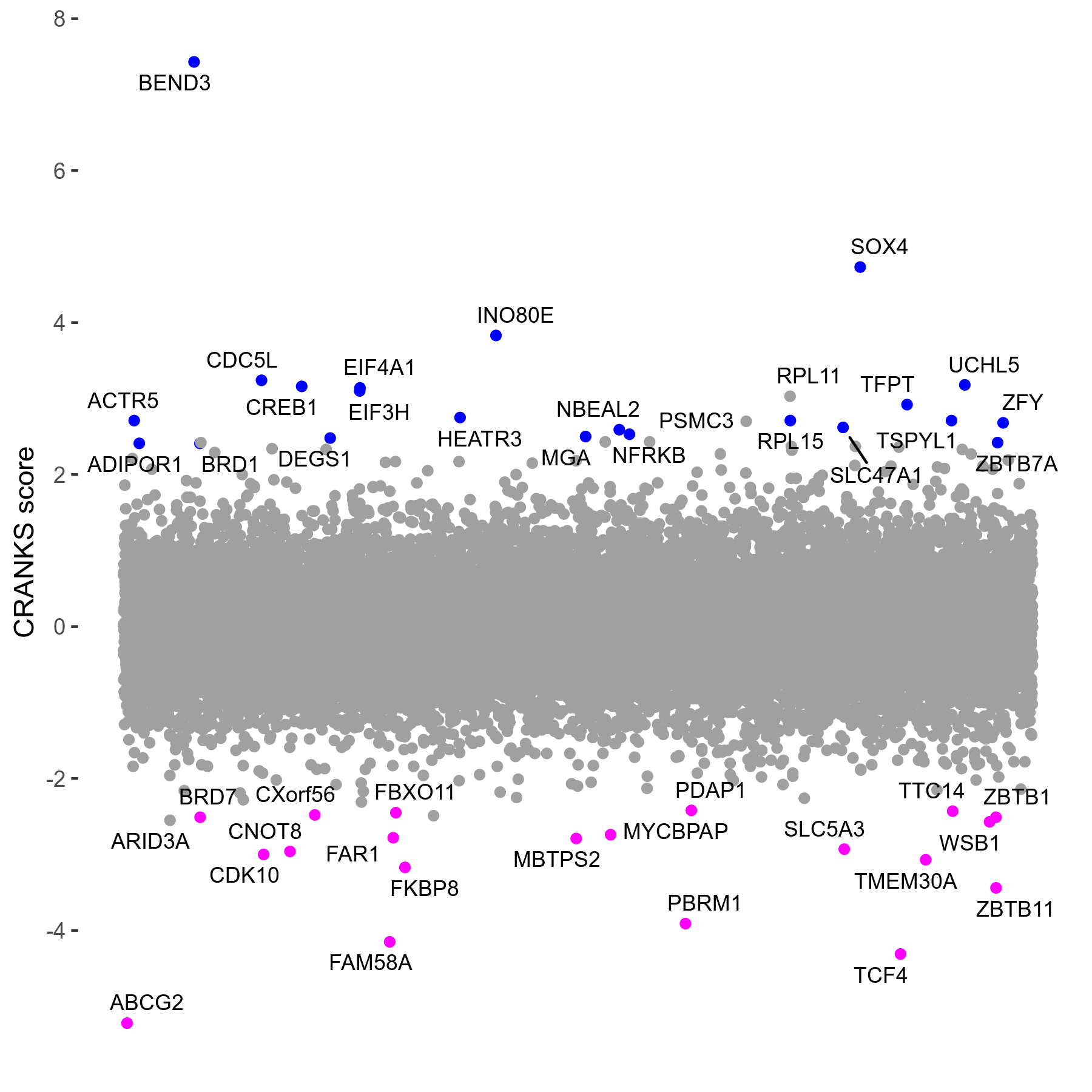
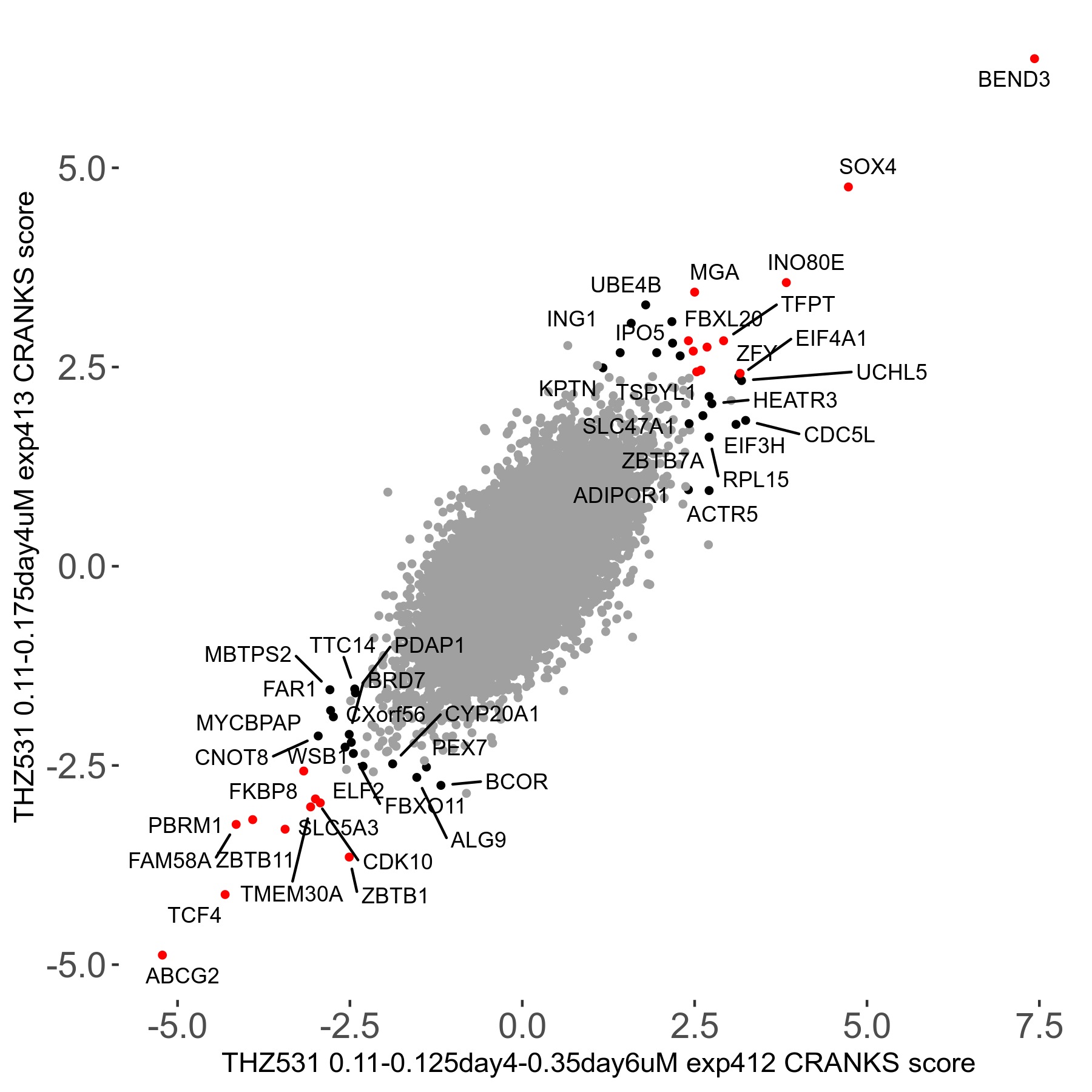
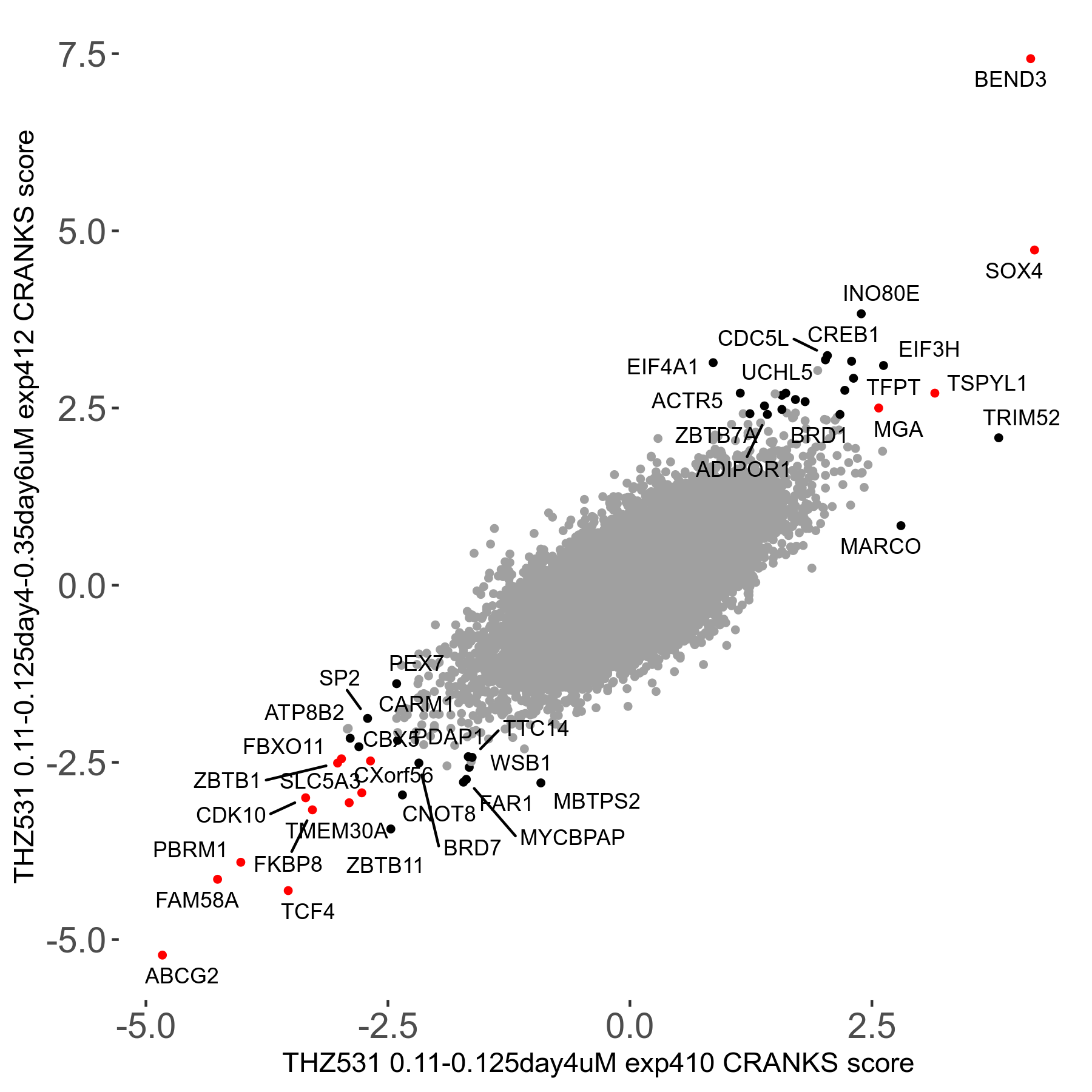
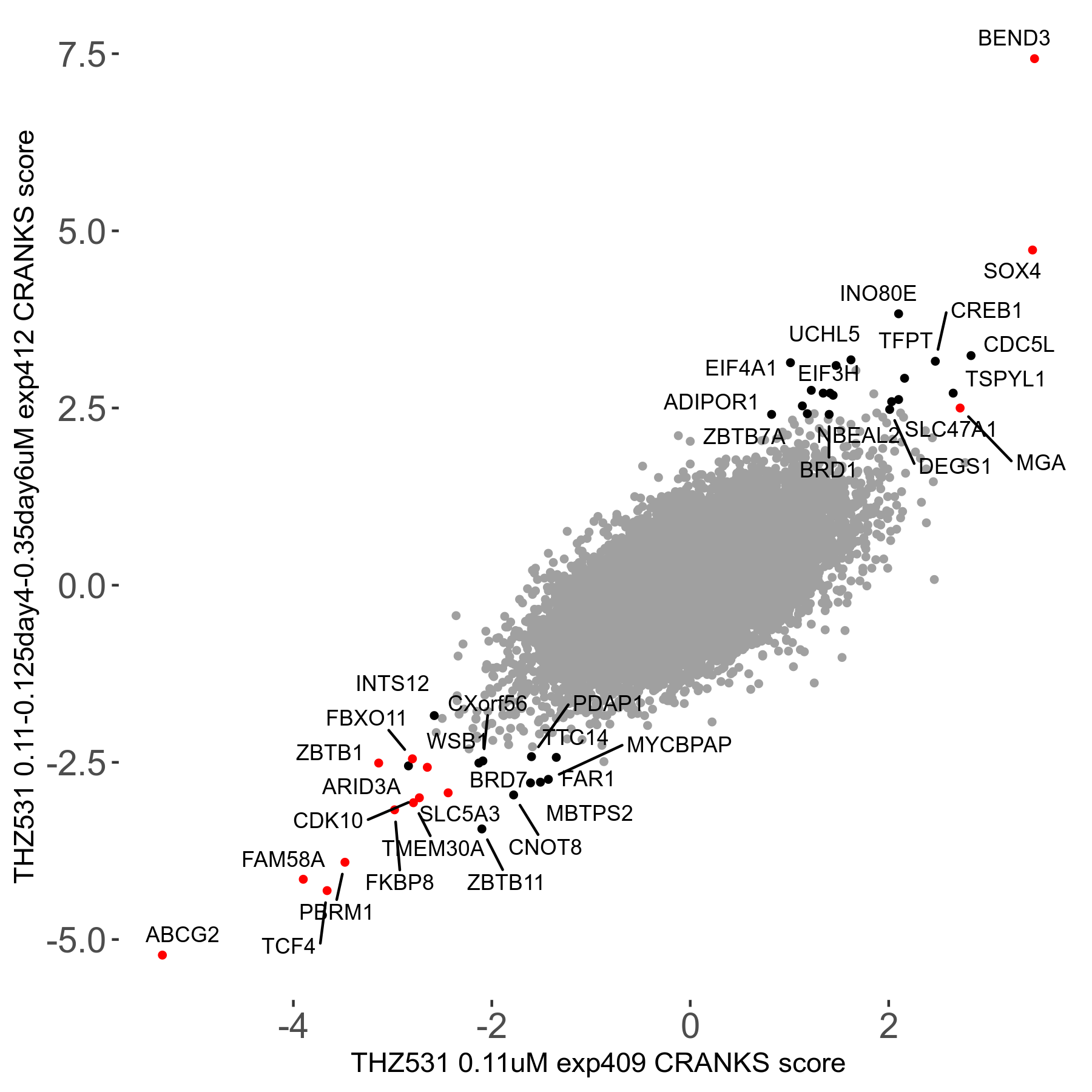
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 20/22 | Scores |