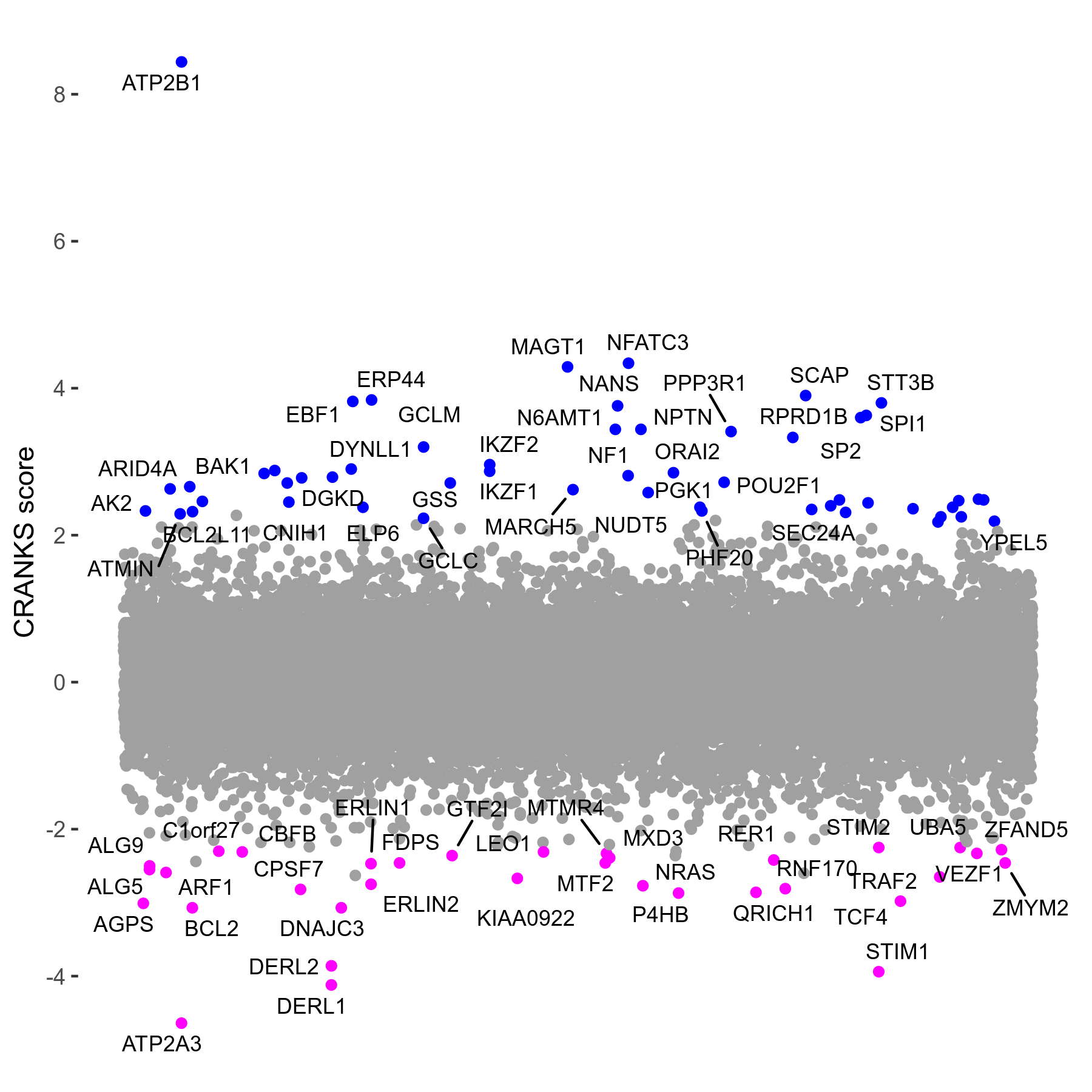
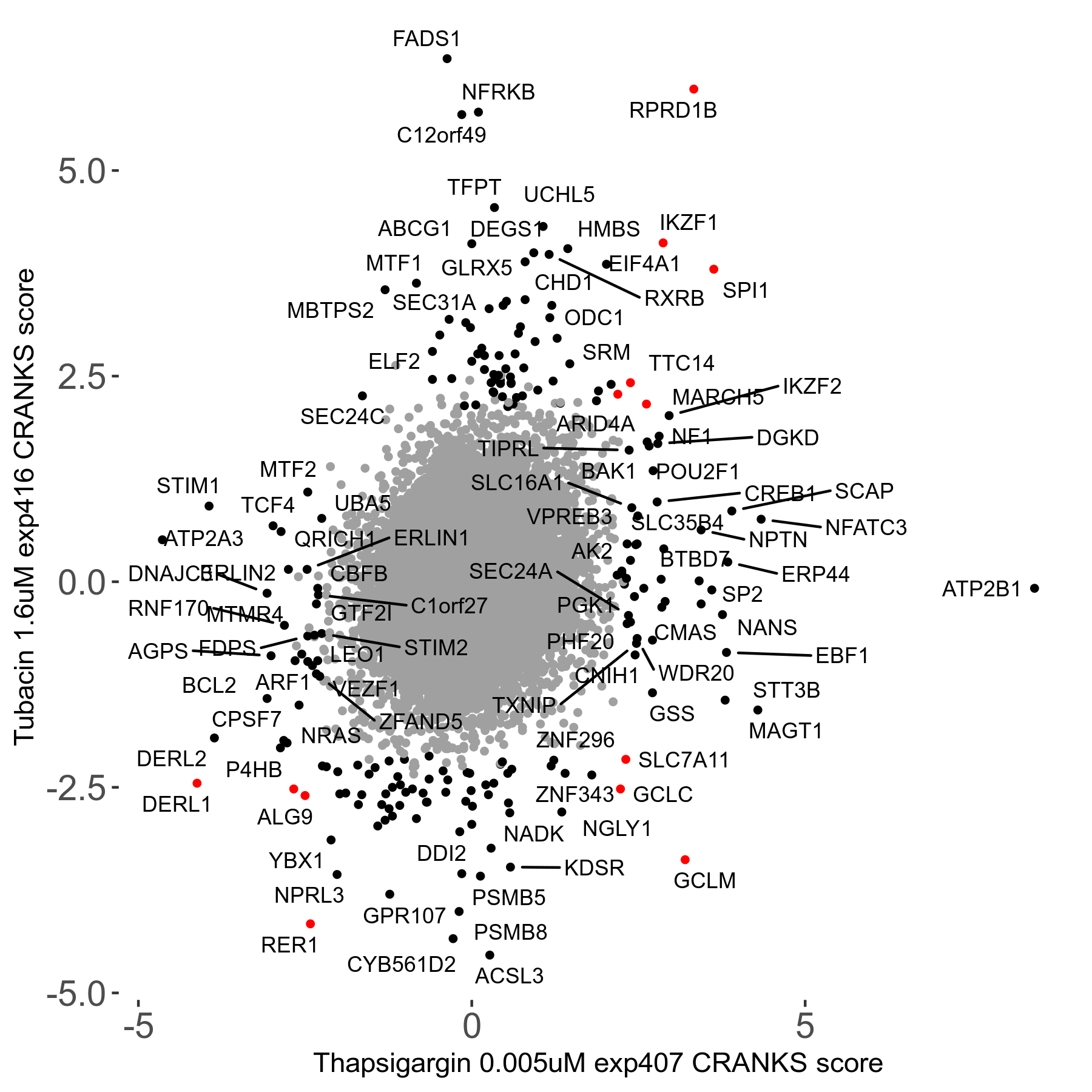
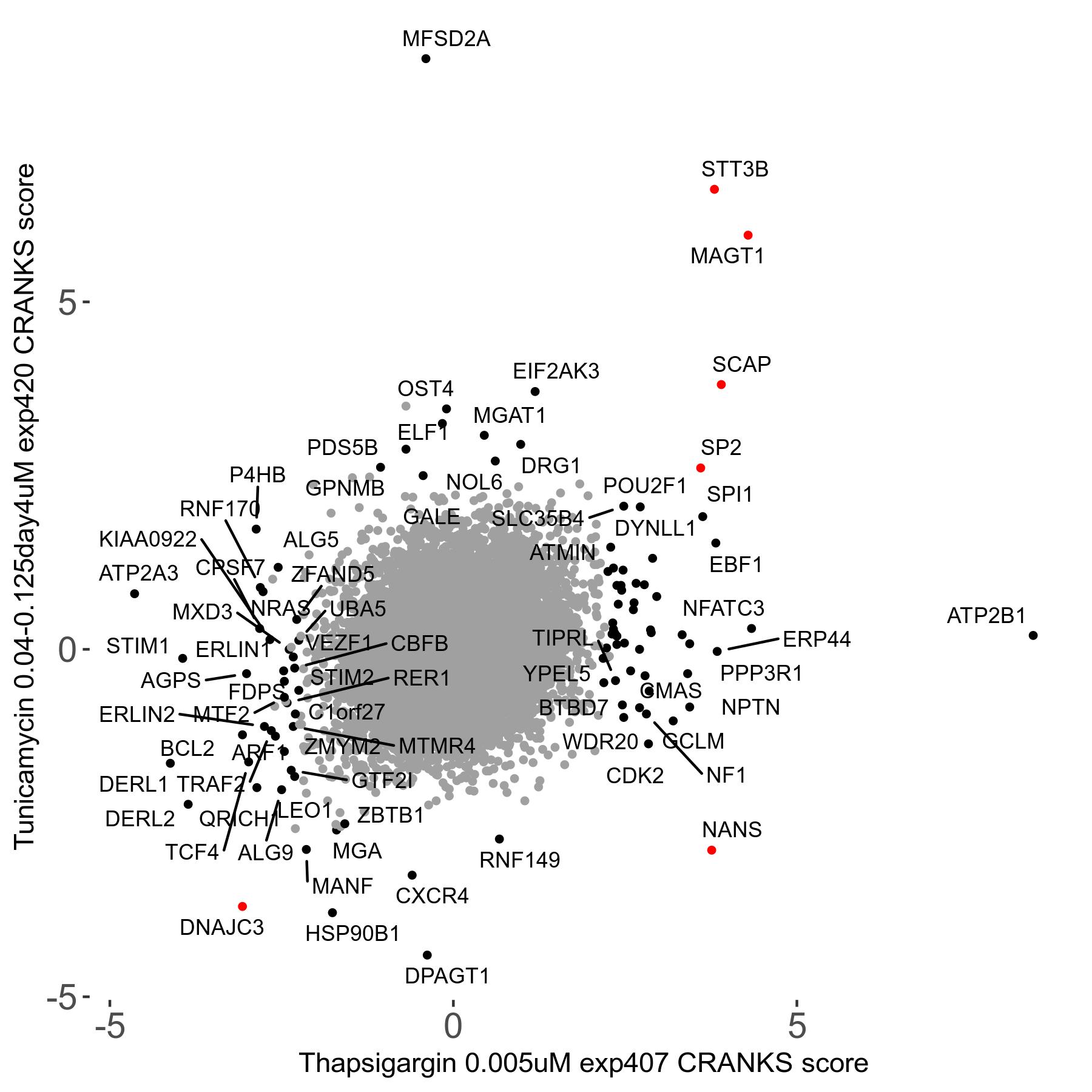
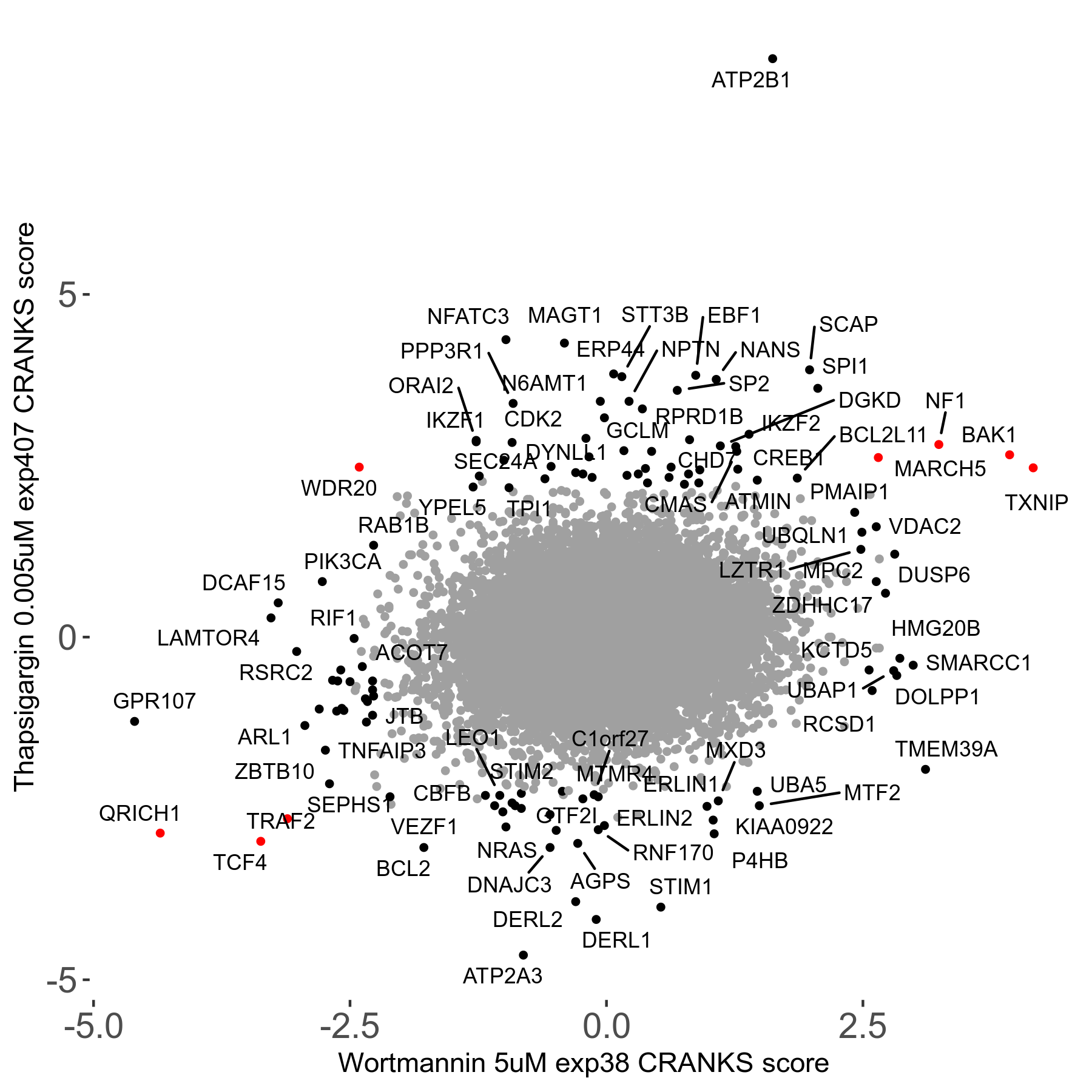
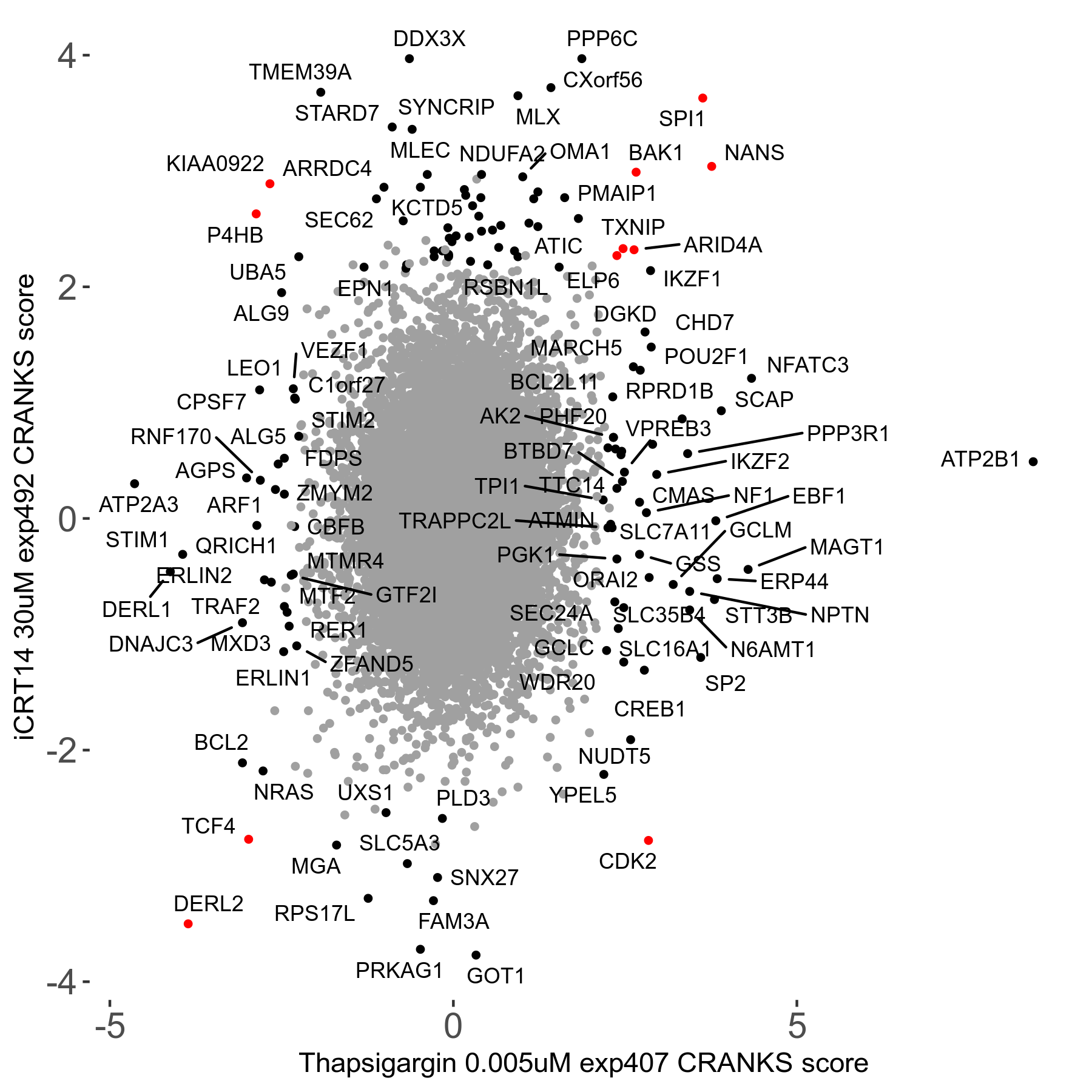
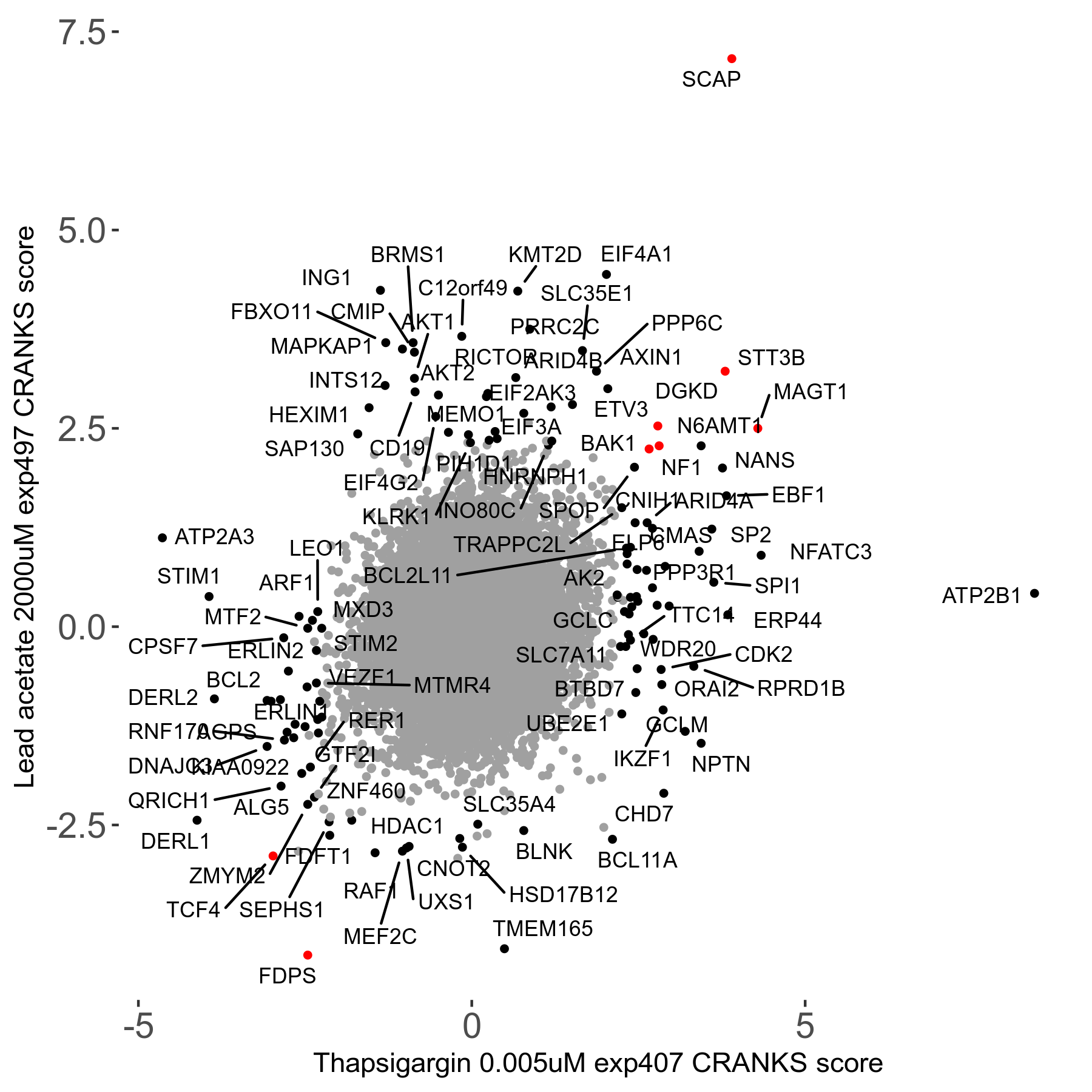
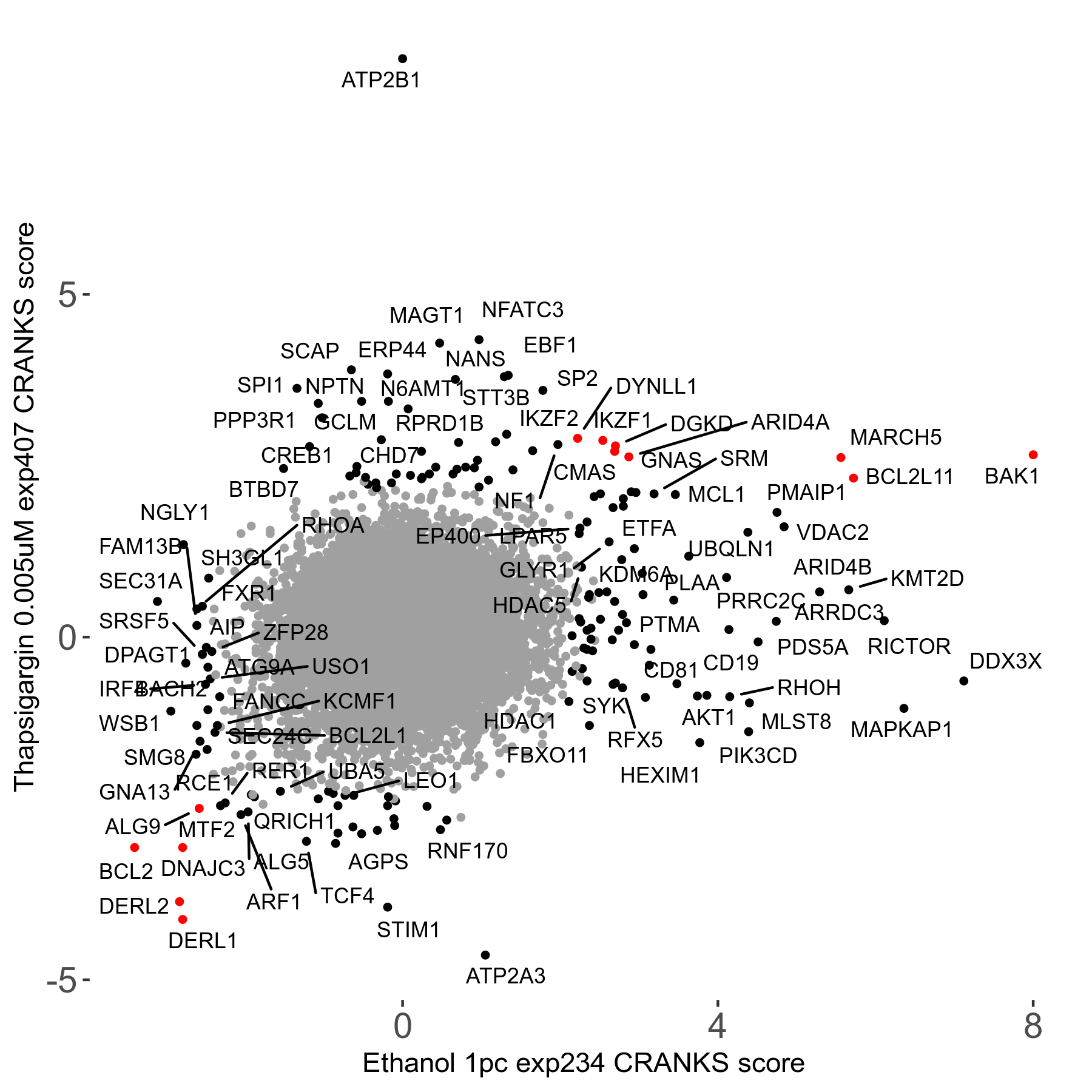
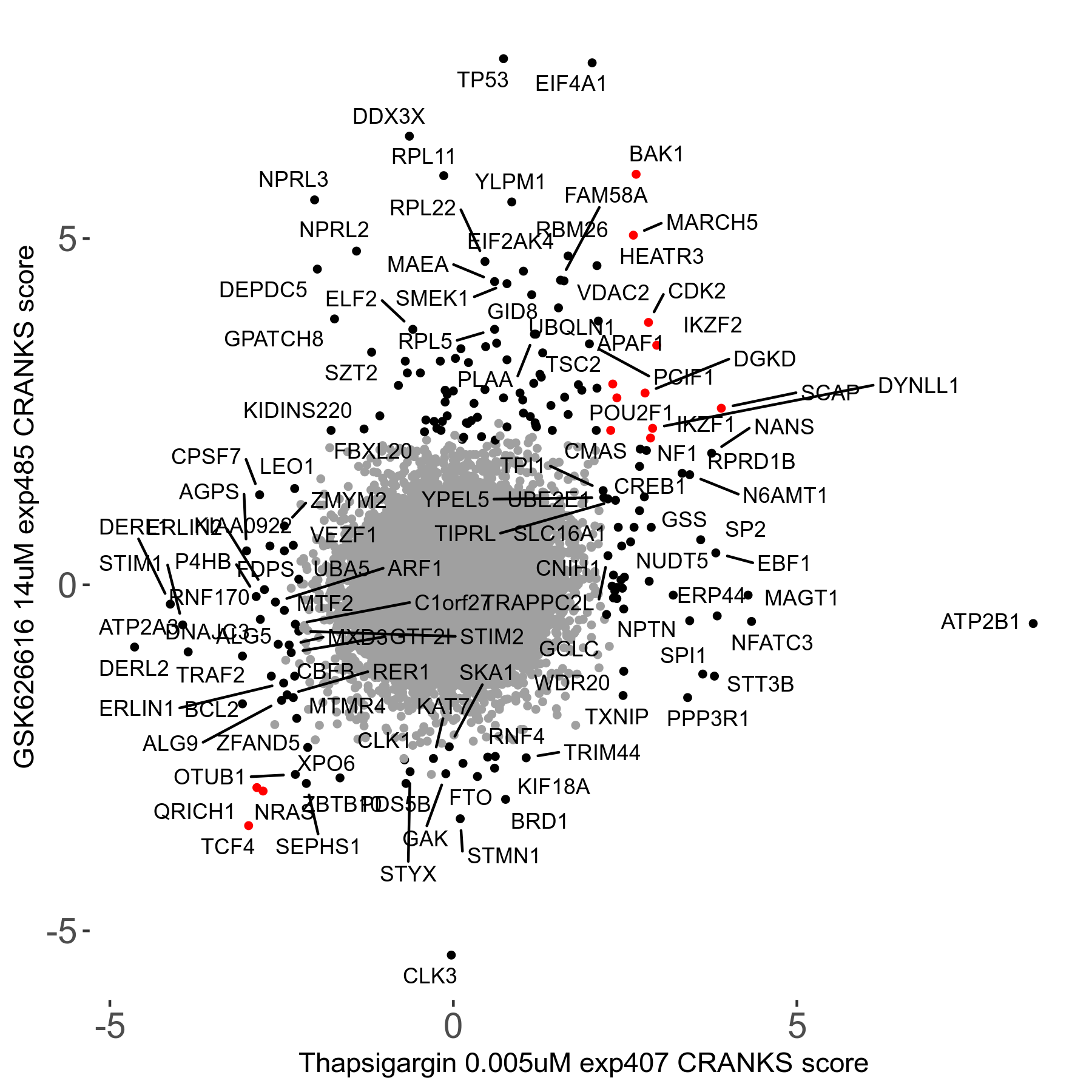
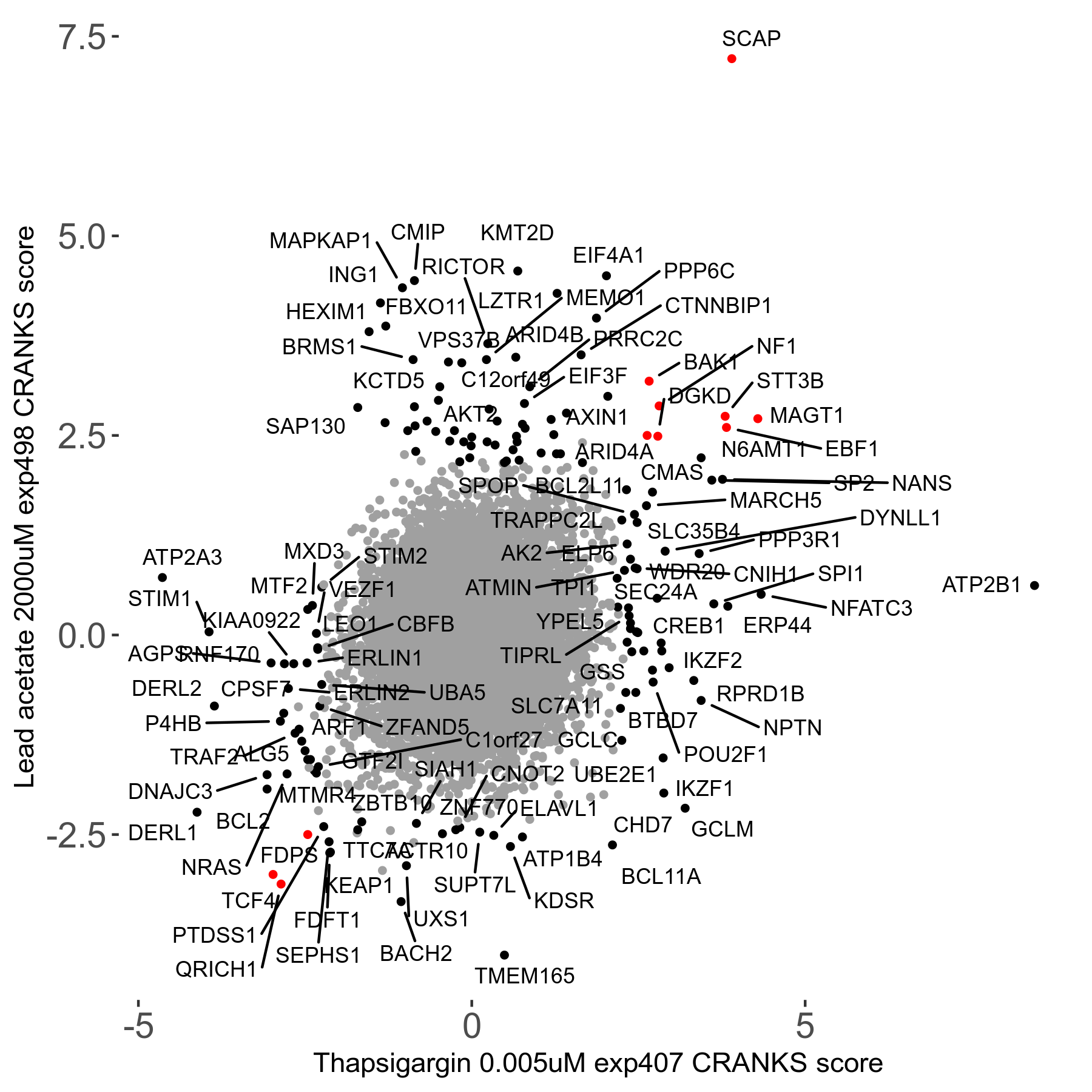
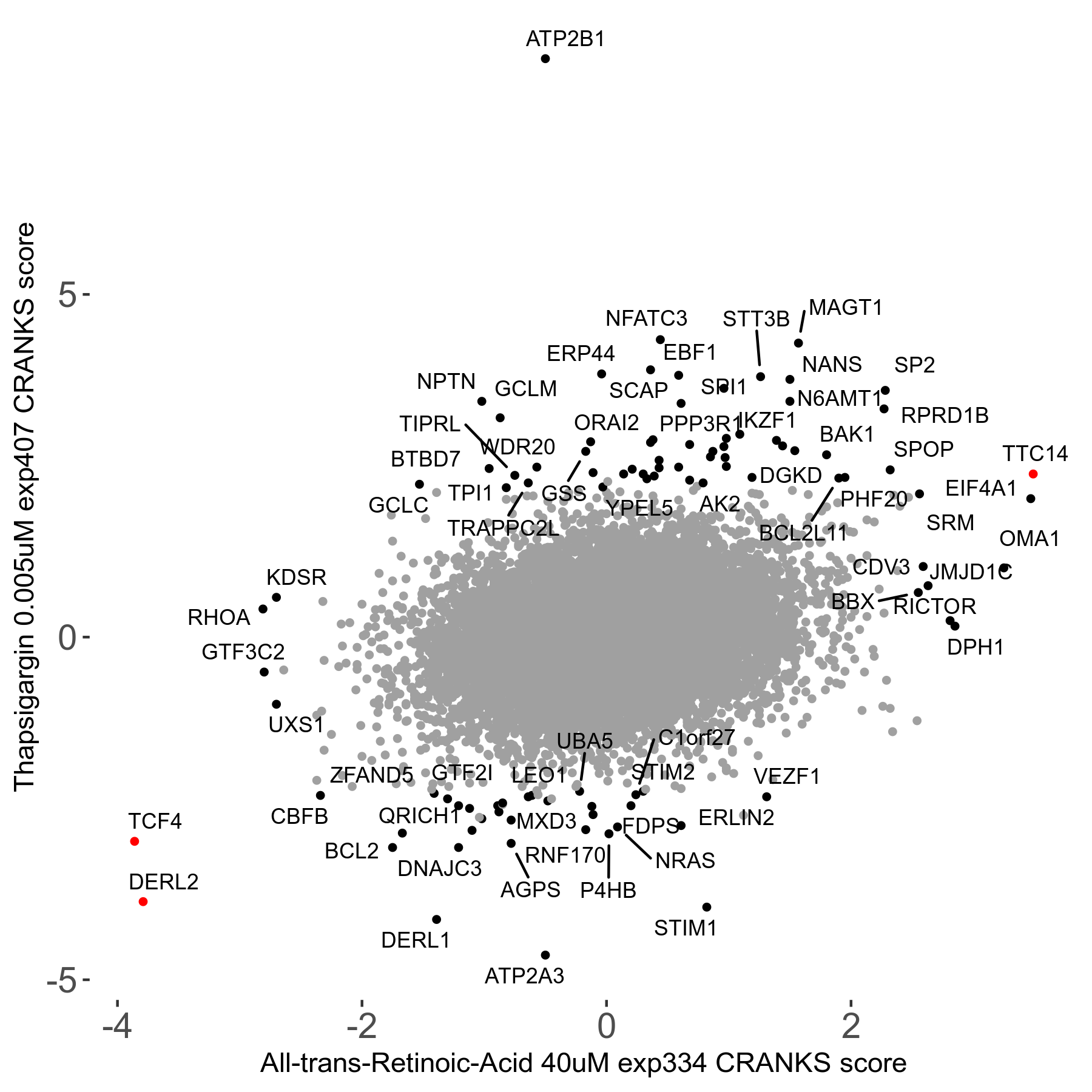
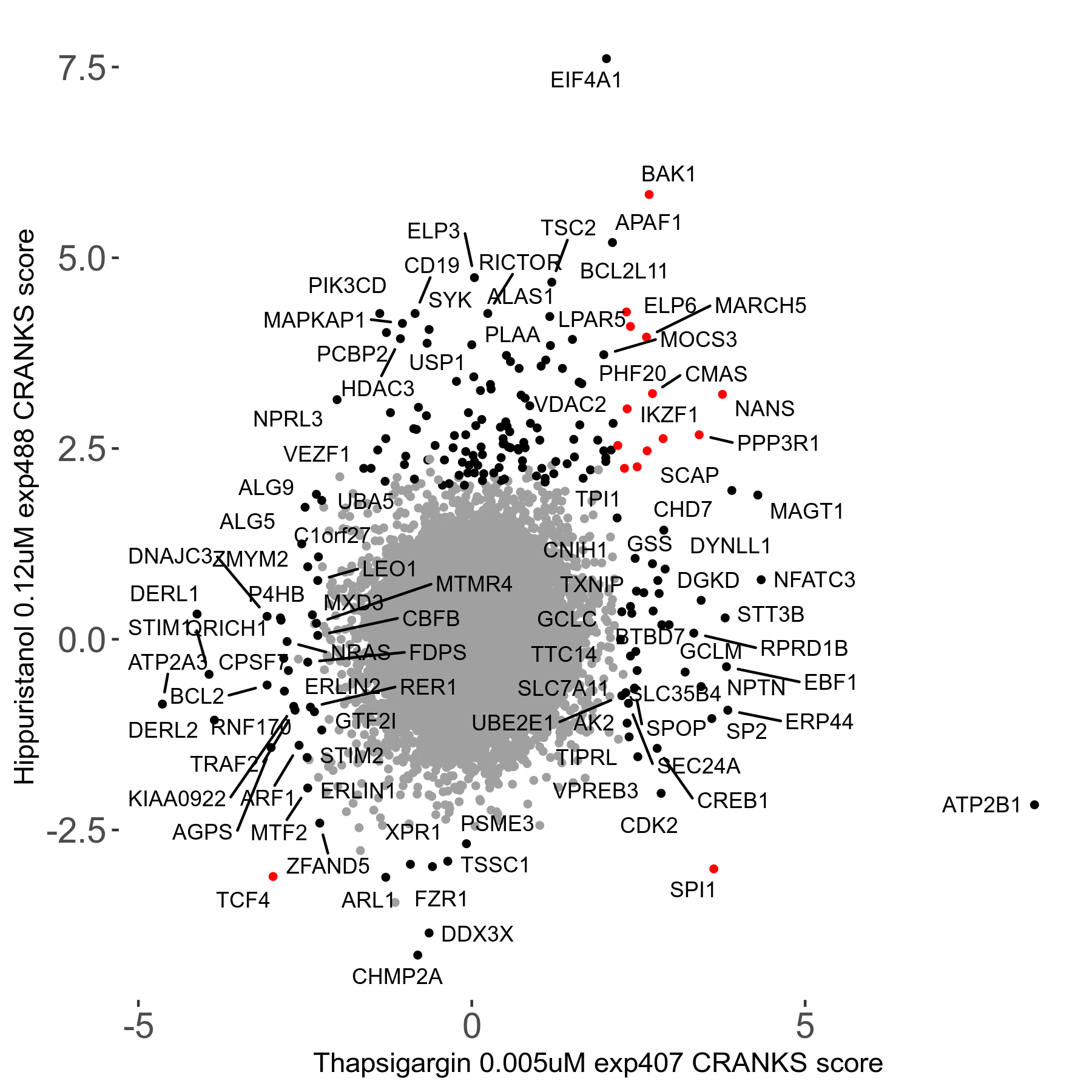
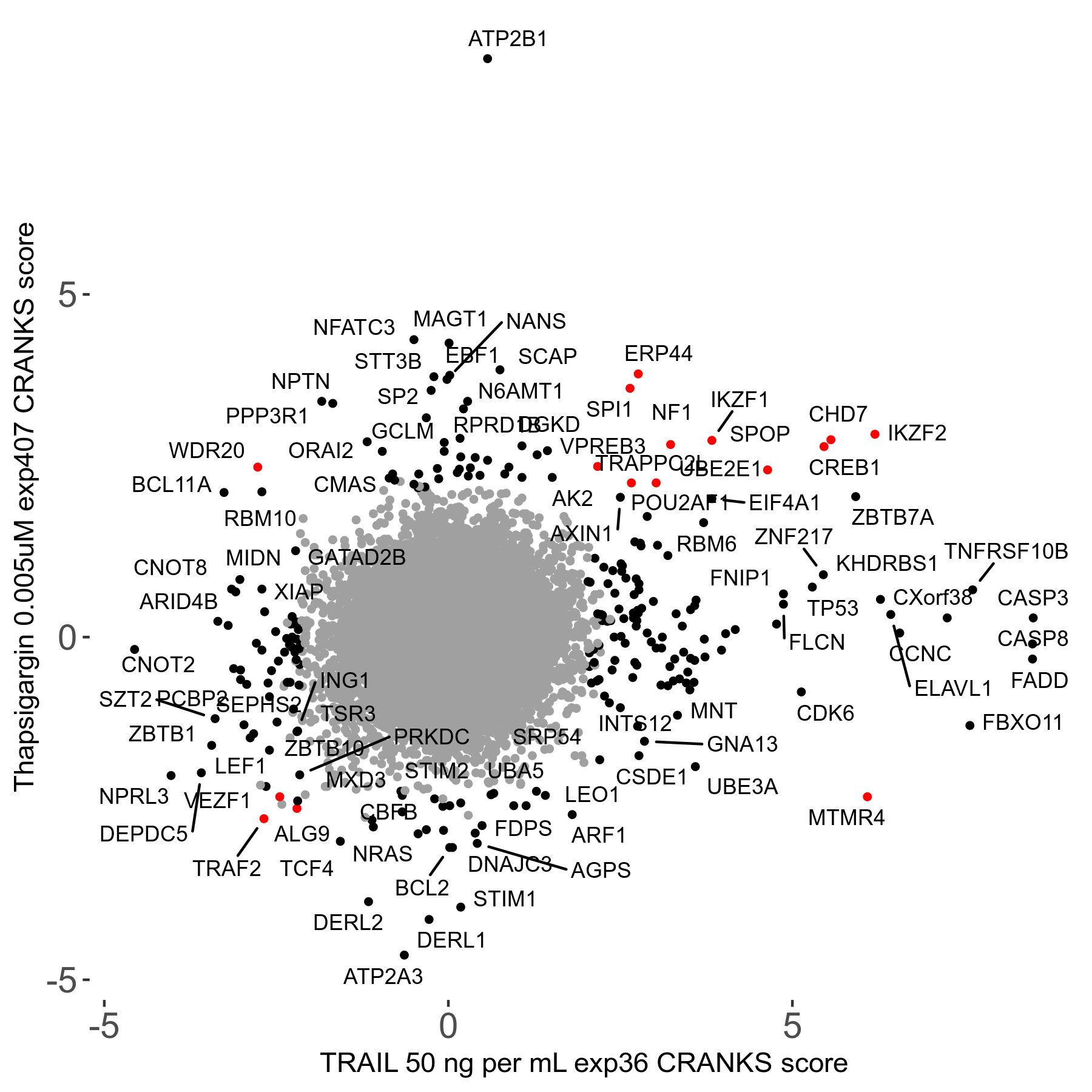
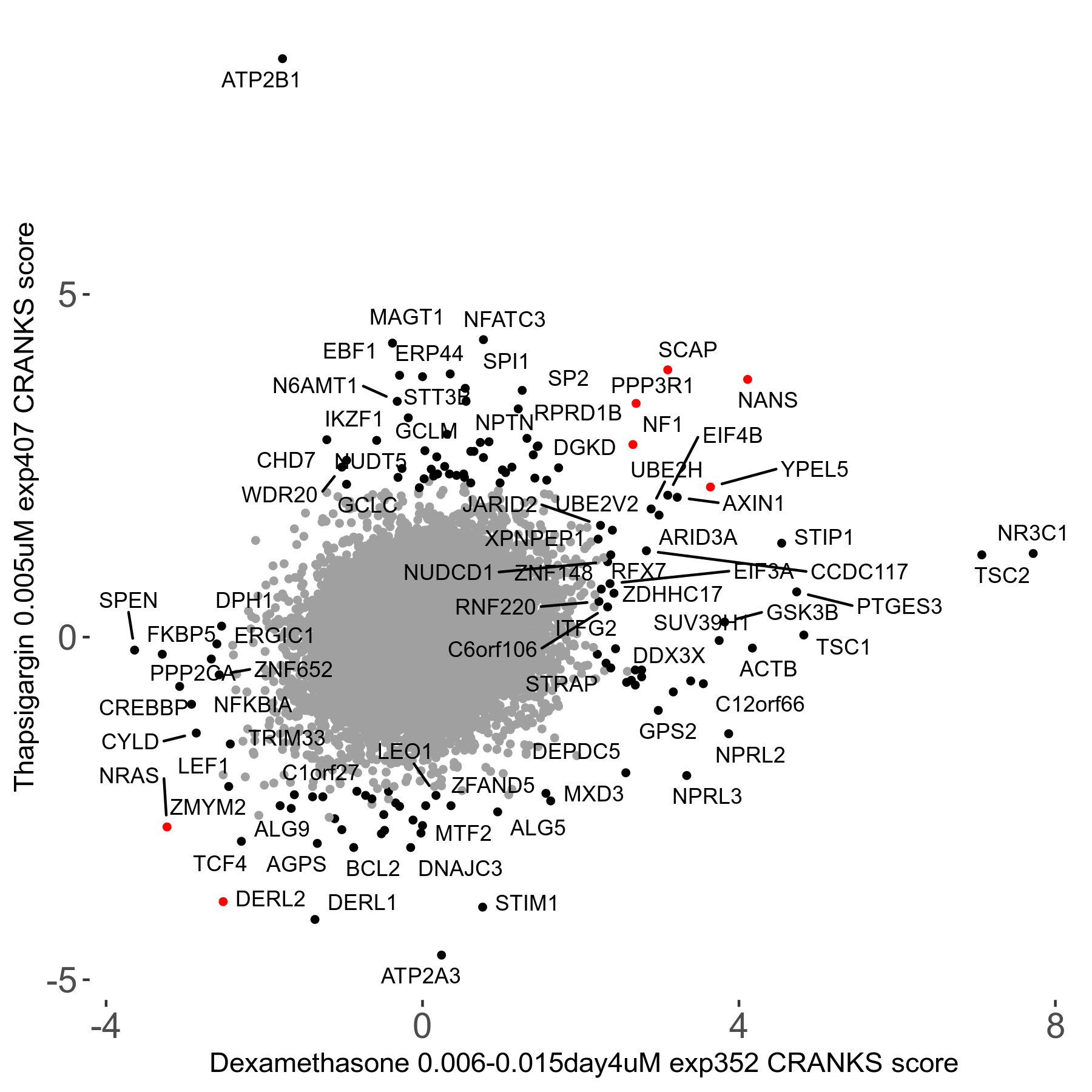
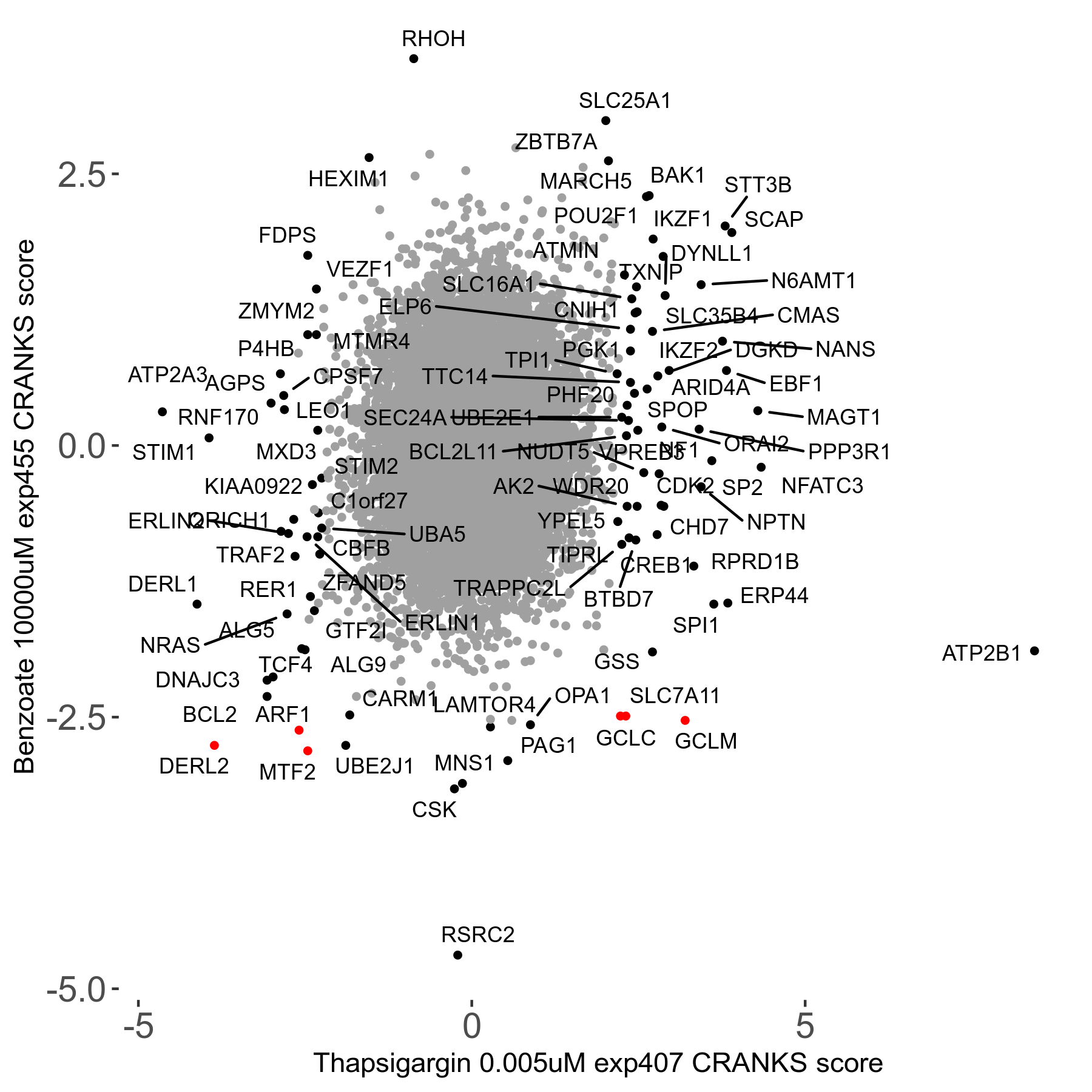
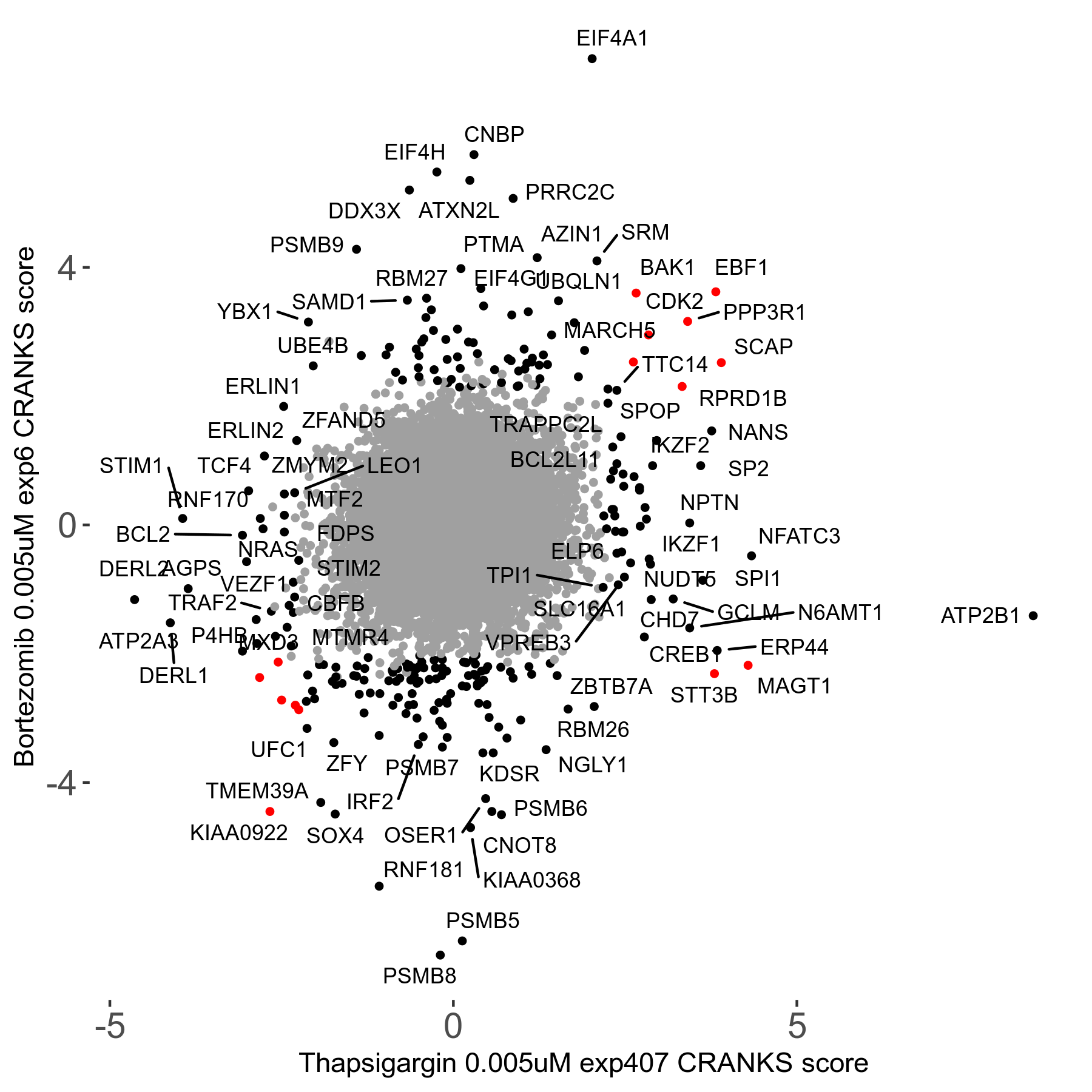
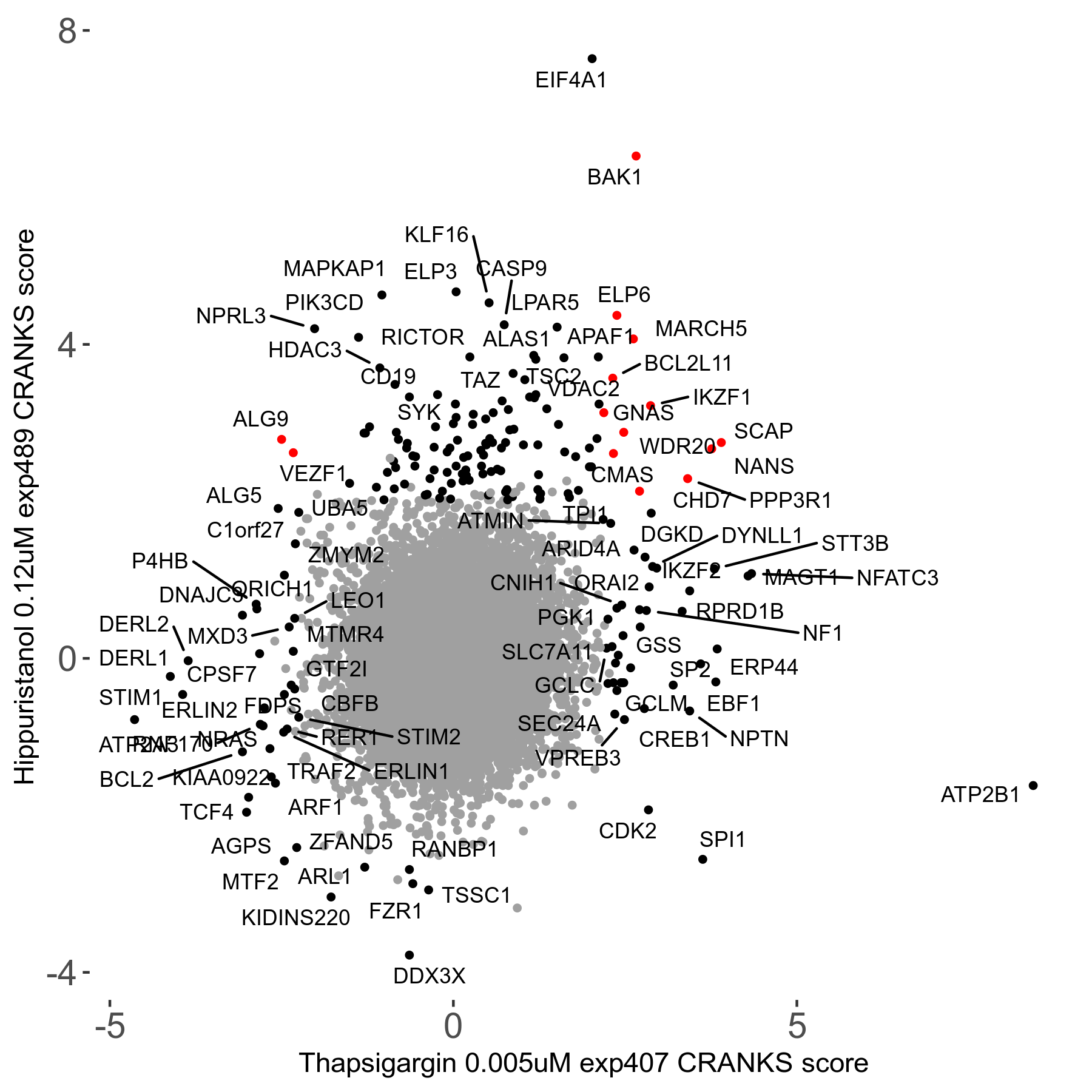
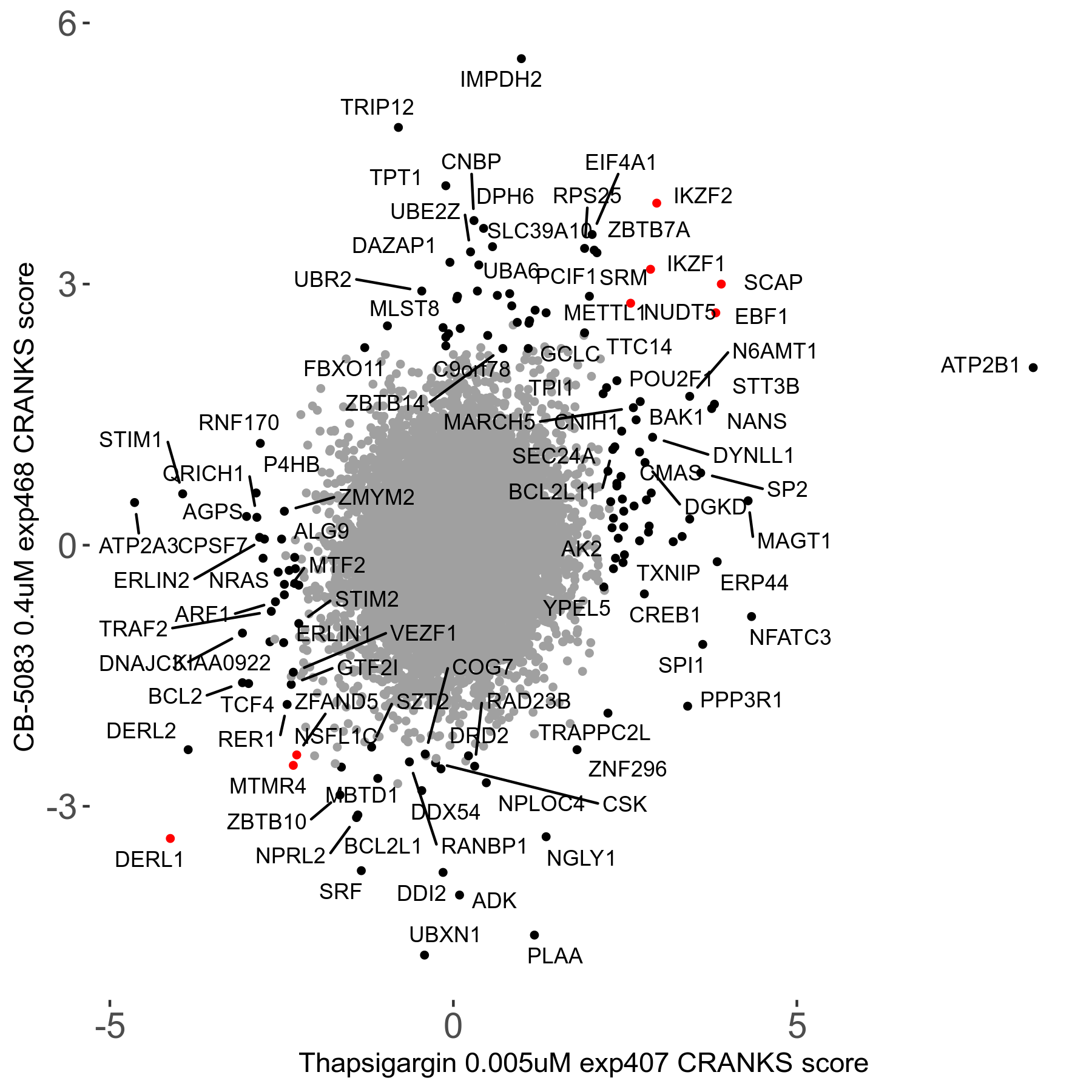
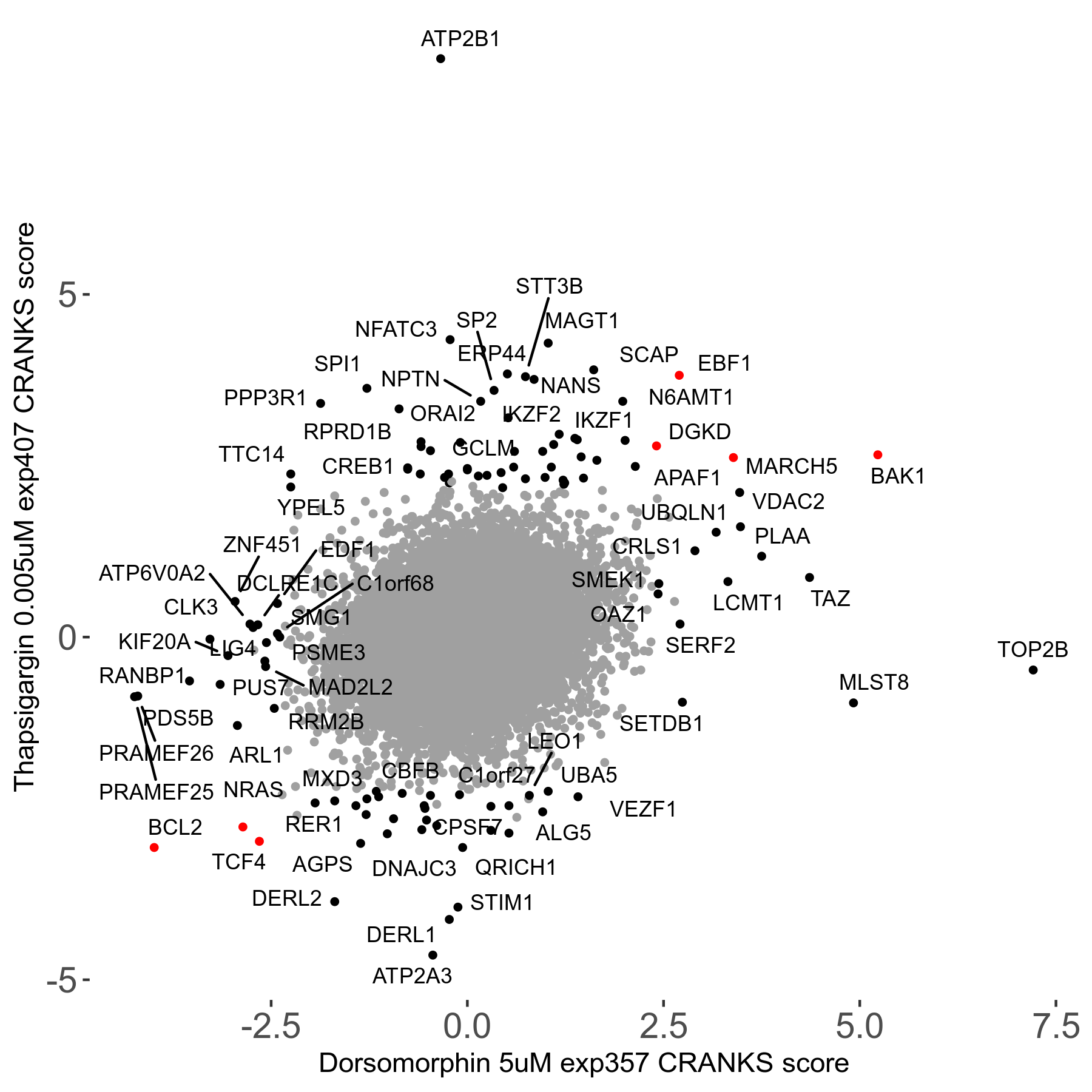
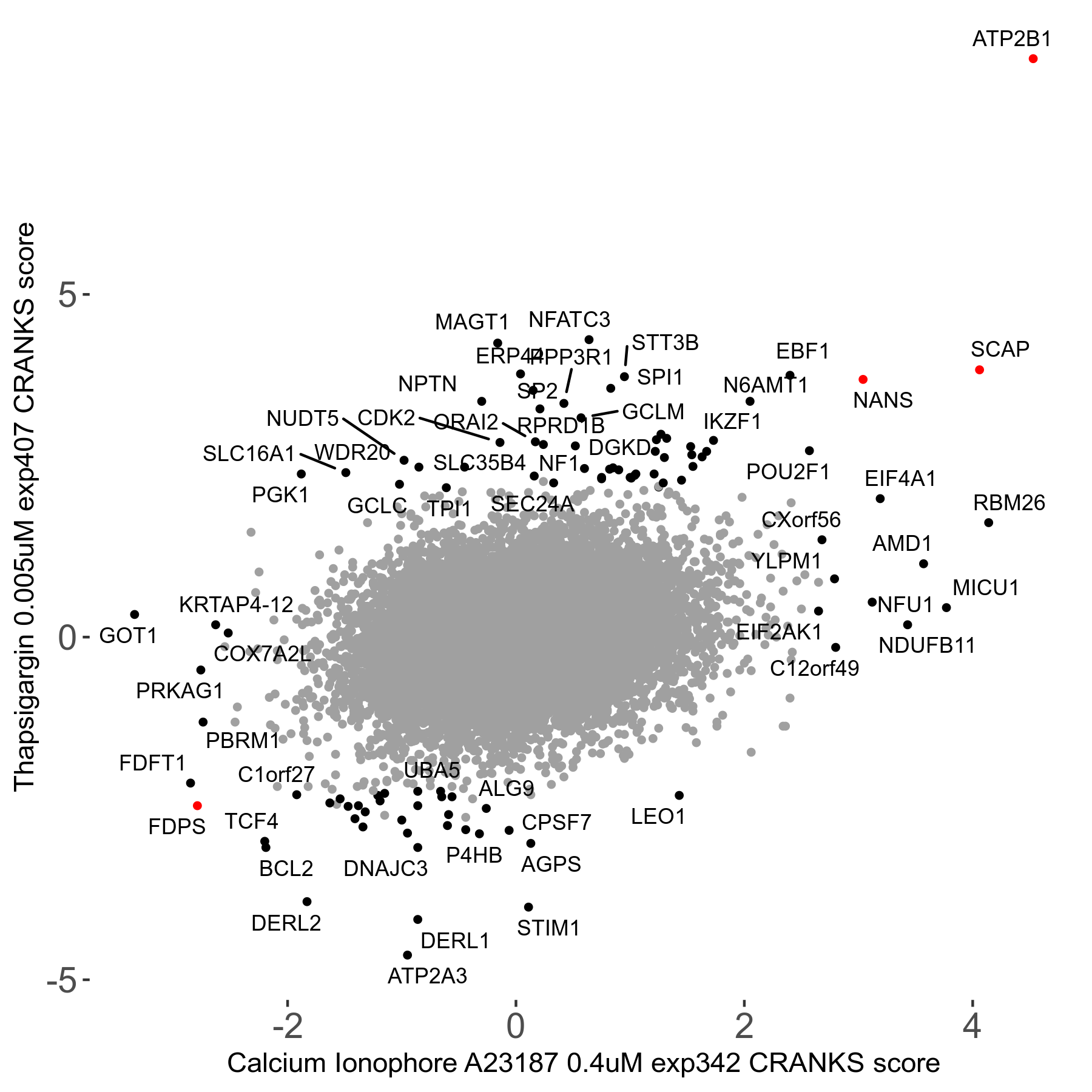
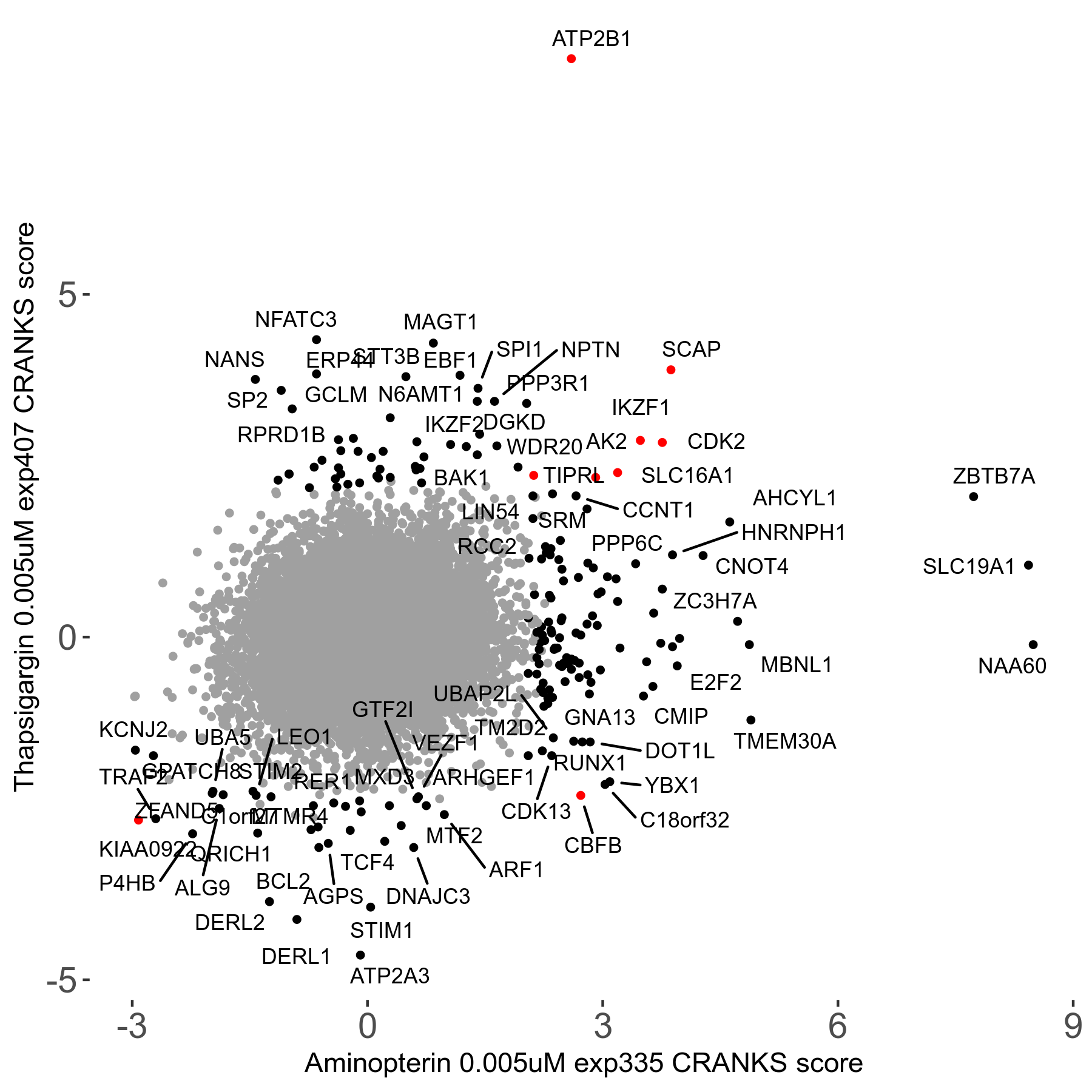
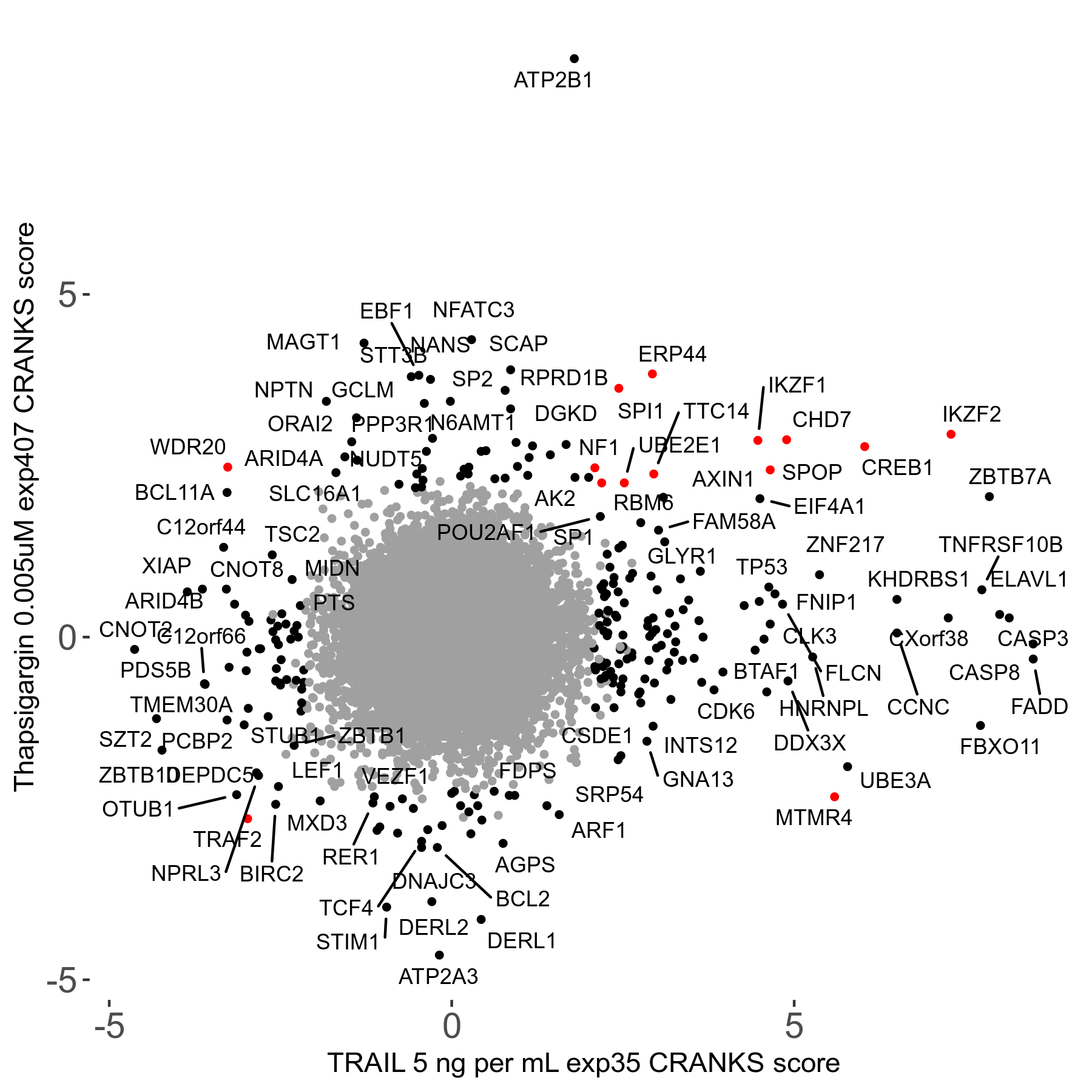
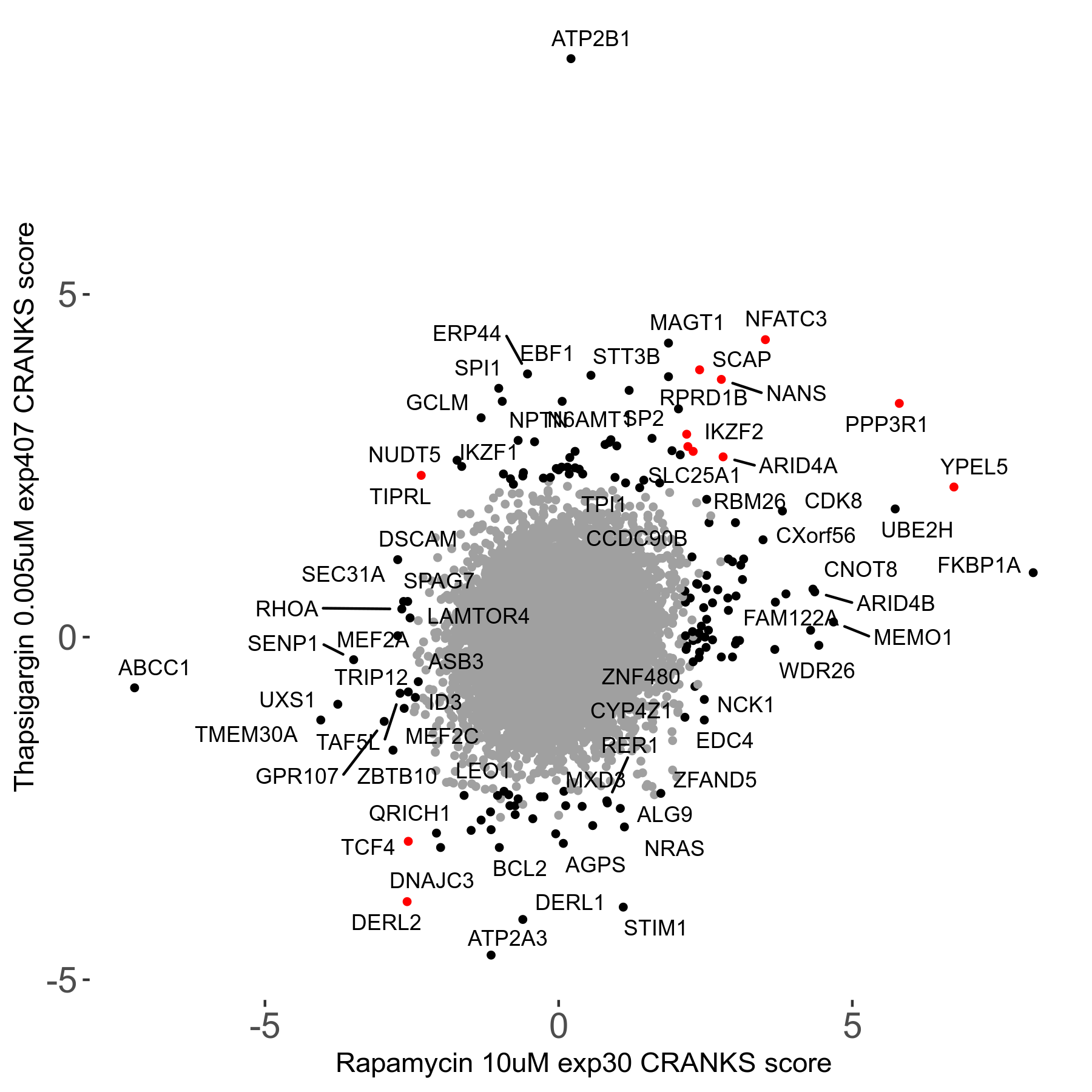
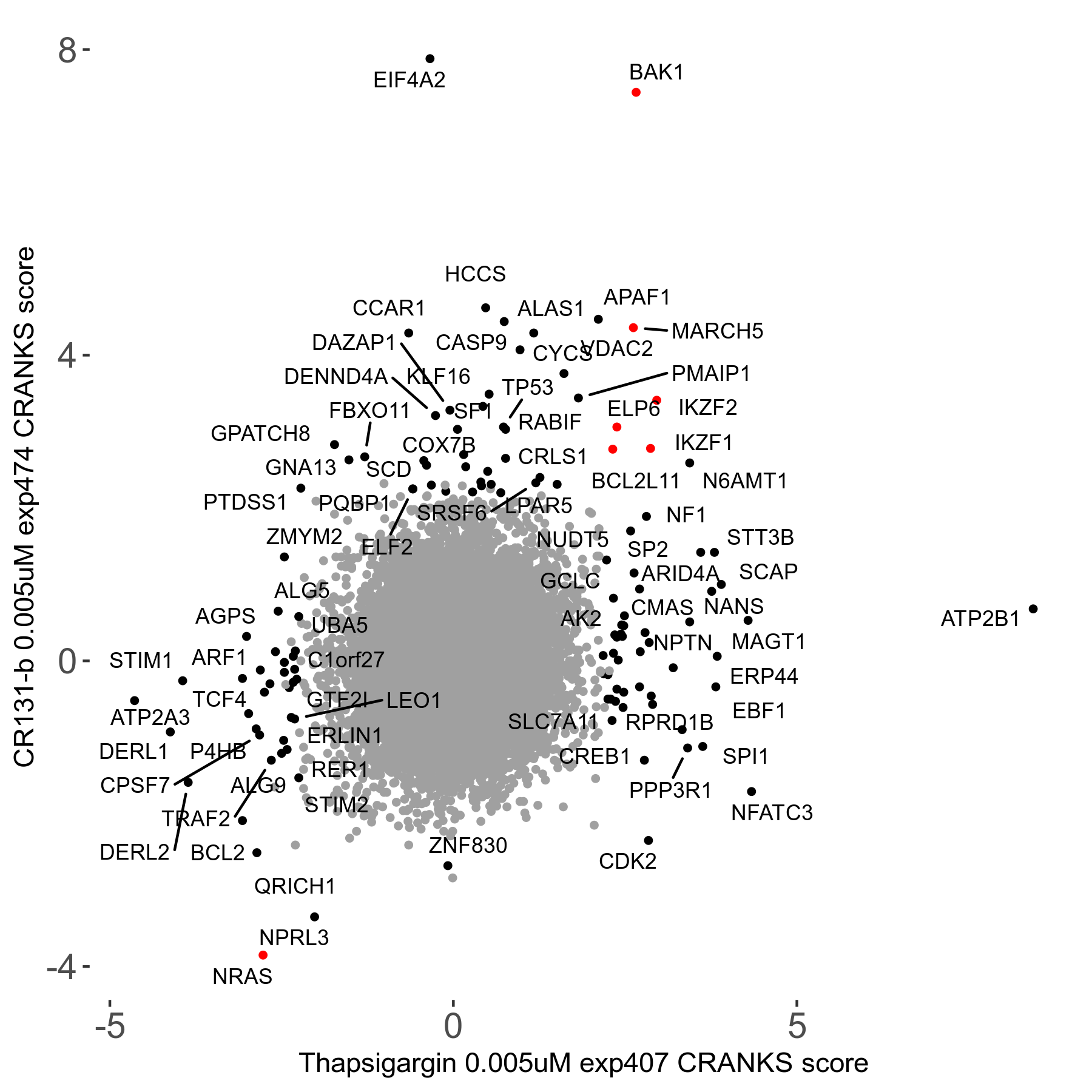
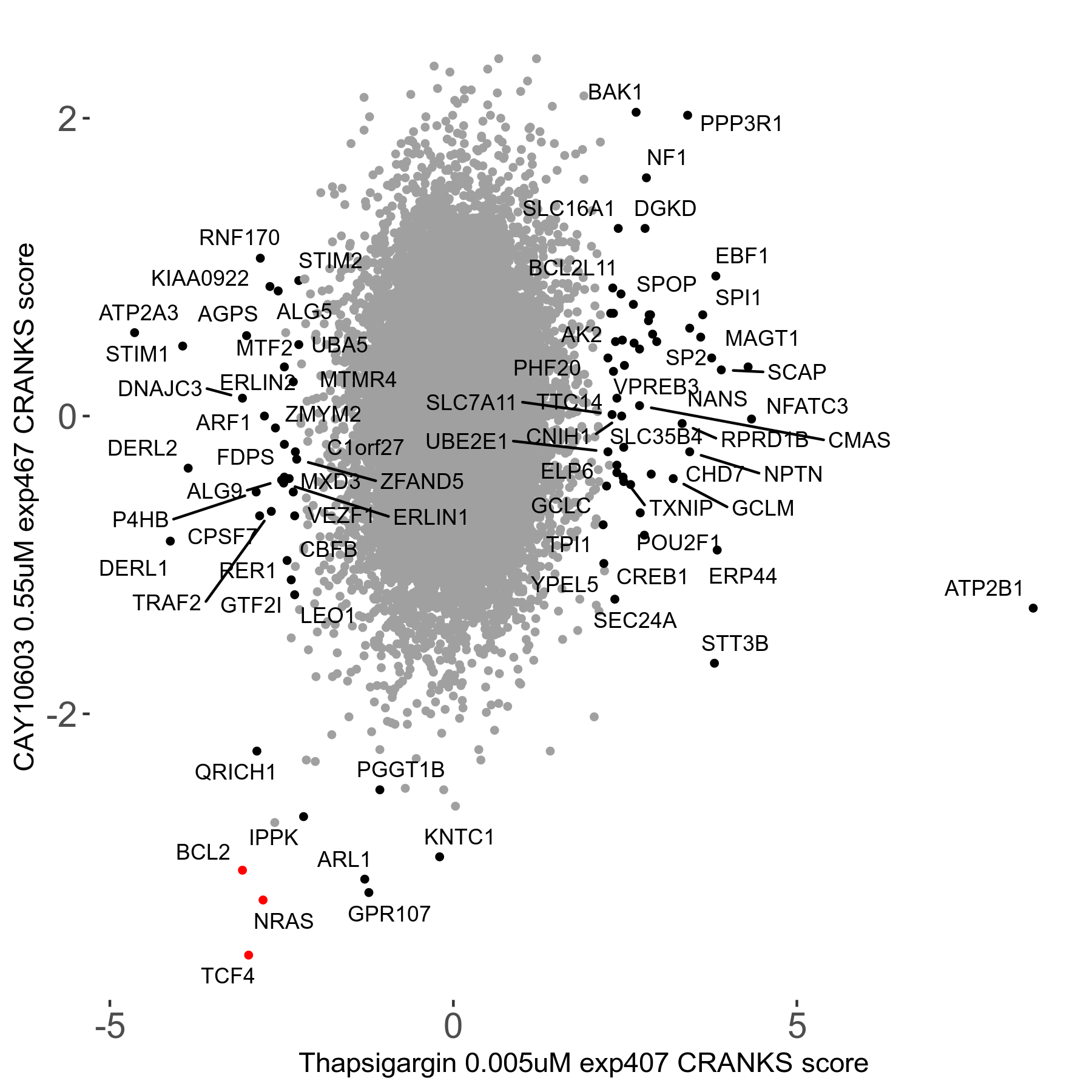
Thapsigargin 0.005μM R07 exp407
Mechanism of Action
Inhibits sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), non-competitive inhibitor, raises intracellular calcium concentration
- Class / Subclass 1: Organelle Function / Membrane Transport Inhibitor
- Class / Subclass 2: Signal Transduction / Ion Channel Inhibitor
Technical Notes
Compound References
- PubChem Name: Thapsigargin
- Synonyms: N/A
- CAS #: 67526-95-8
- PubChem CID: 446378
- IUPAC: [(3S,3aR,4S,6S,6aR,7S,8S,9bS)-6-acetyloxy-4-butanoyloxy-3,3a-dihydroxy-3,6,9-trimethyl-8-[(Z)-2-methylbut-2-enoyl]oxy-2-oxo-4,5,6a,7,8,9b-hexahydroazuleno[4,5-b]furan-7-yl] octanoate
- INCHI Name: InChI=1S/C34H50O12/c1-9-12-13-14-15-17-24(37)43-28-26-25(20(5)27(28)44-30(38)19(4)11-3)29-34(41,33(8,40)31(39)45-29)22(42-23(36)16-10-2)18-32(26,7)46-21(6)35/h11,22,26-29,40-41H,9-10,12-18H2,1-8H3/b19-11-/t22-,26+,27-,28-,29-,32-,33+,34+/m0/s1
- INCHI Key: IXFPJGBNCFXKPI-FSIHEZPISA-N
- Molecular Weight: 650.8
- Canonical SMILES: CCCCCCCC(=O)OC1C2C(=C(C1OC(=O)C(=CC)C)C)C3C(C(CC2(C)OC(=O)C)OC(=O)CCC)(C(C(=O)O3)(C)O)O
- Isomeric SMILES: CCCCCCCC(=O)O[C@H]1[C@H]2C(=C([C@@H]1OC(=O)/C(=C\\C)/C)C)[C@H]3[C@]([C@H](C[C@]2(C)OC(=O)C)OC(=O)CCC)([C@](C(=O)O3)(C)O)O
- Molecular Formula: C34H50O12
Compound Supplier
- Supplier Name: Cayman Chemical
- Catalog #: 10522
- Lot #: 0507161-12
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+Na)+ Calcd for C34H50O12 673.31945; found 673.32042
Dose Response Curve
- Platform ID: Thapsigargin
- Min: 14.5736; Max: 99.8187

| IC | Concentration (µM) |
|---|---|
| IC10 | N/A |
| IC20 | N/A |
| IC30 | 0.0032 |
| IC40 | 0.0061 |
| IC50 | 0.0103 |
| IC60 | N/A |
| IC70 | N/A |
| IC80 | N/A |
| IC90 | N/A |
Screen Summary
- Round: 07
- Dose: 5nM
- Days of incubation: 8
- Doublings: 3.4
- Numbers of reads: 22303546
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 34/54 | Scores |