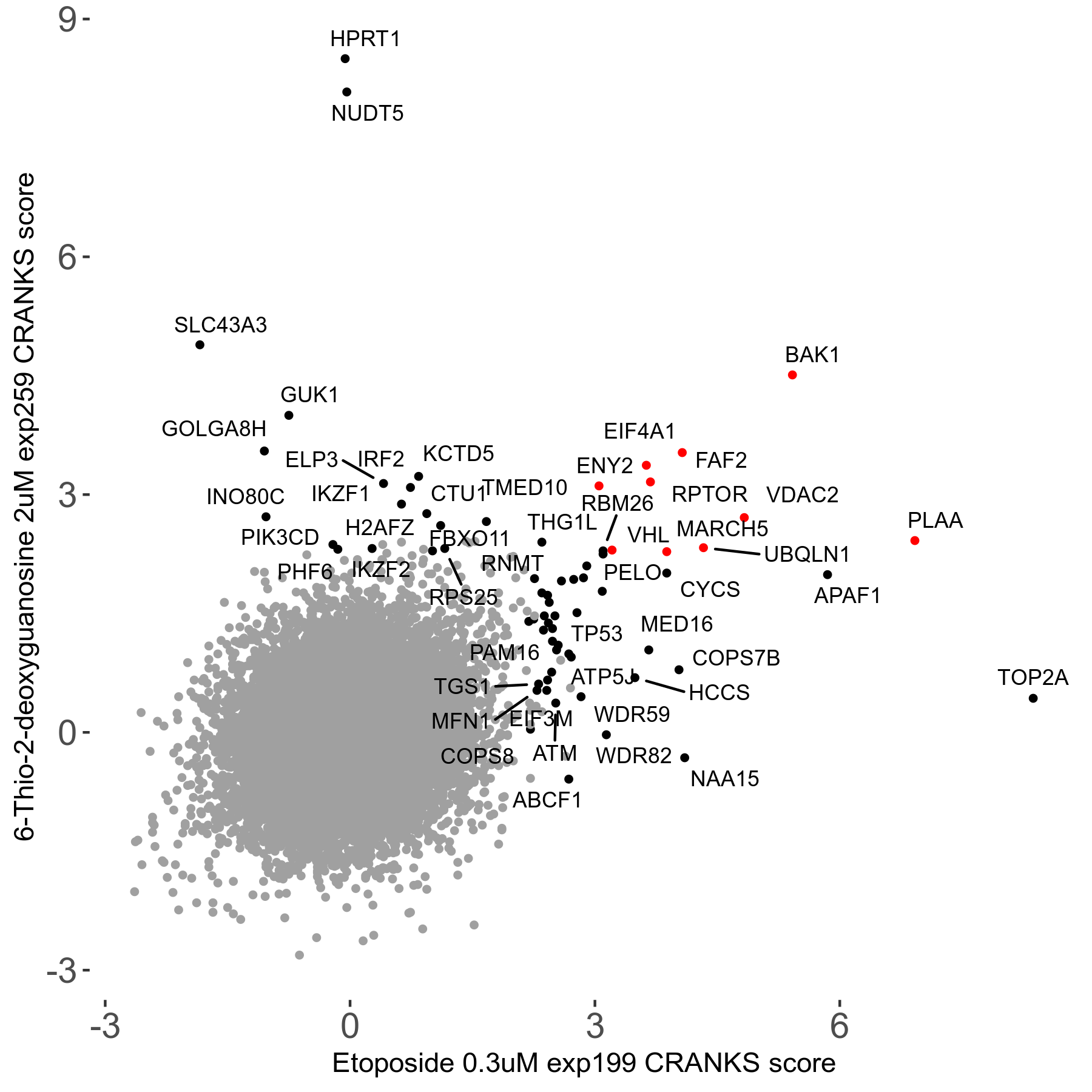
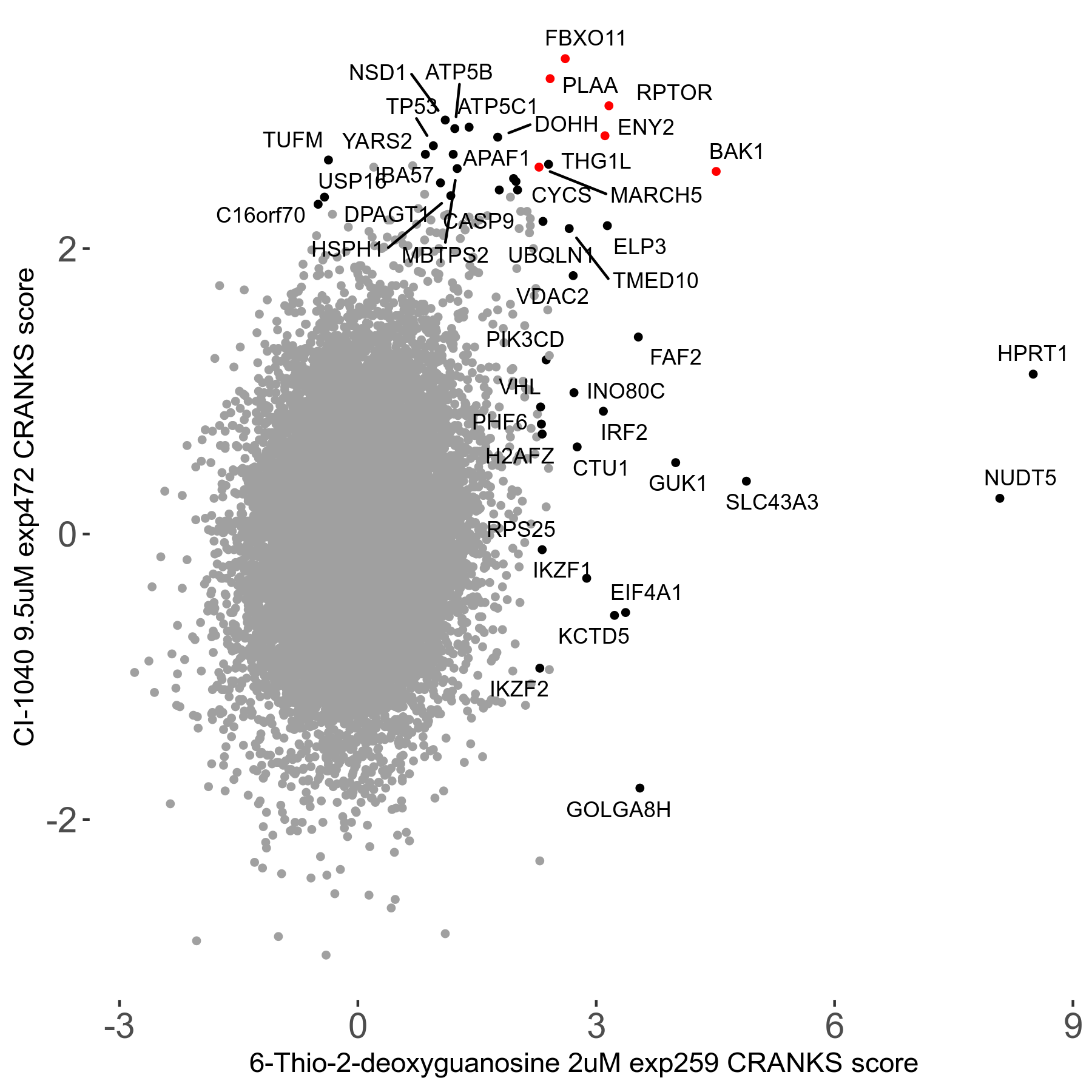
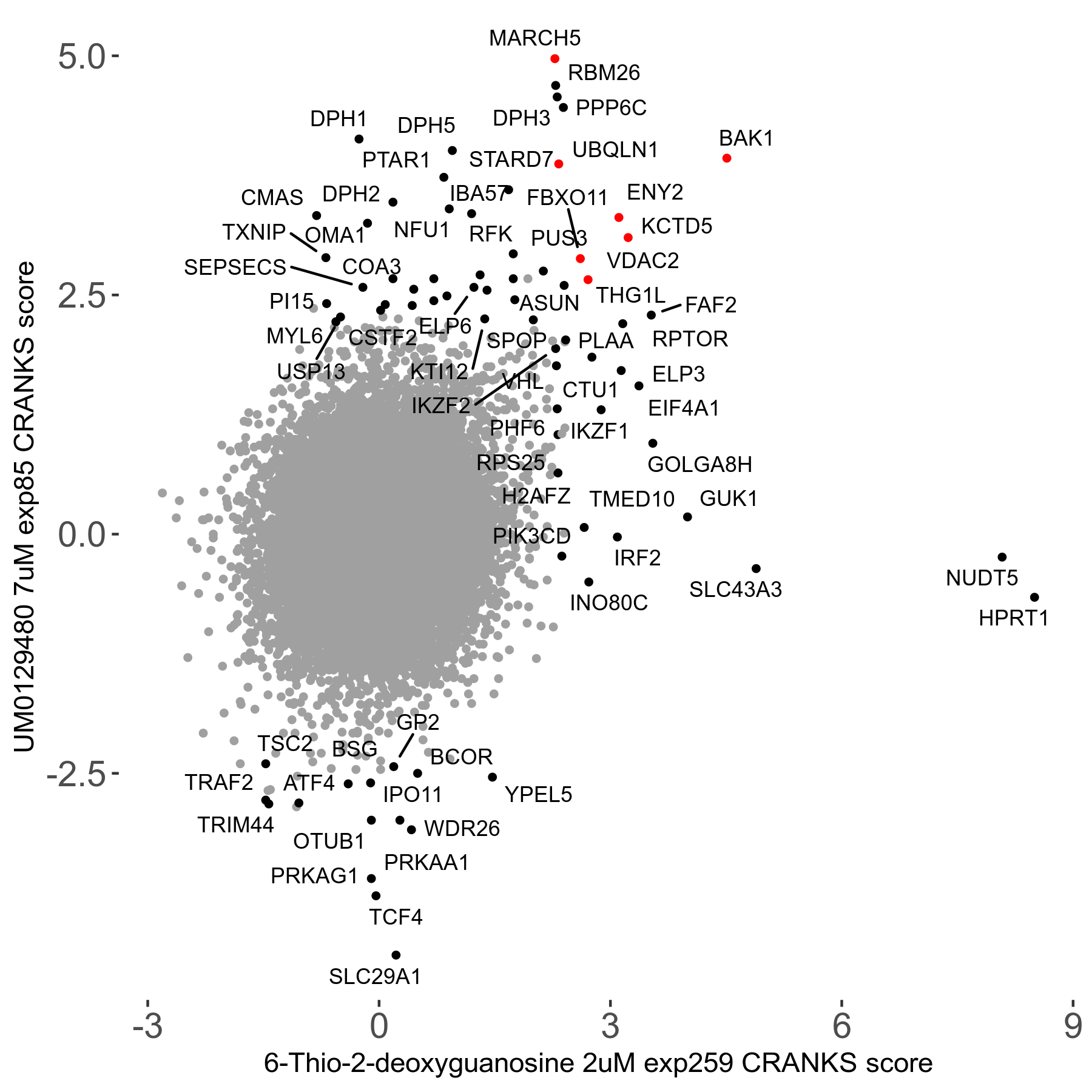
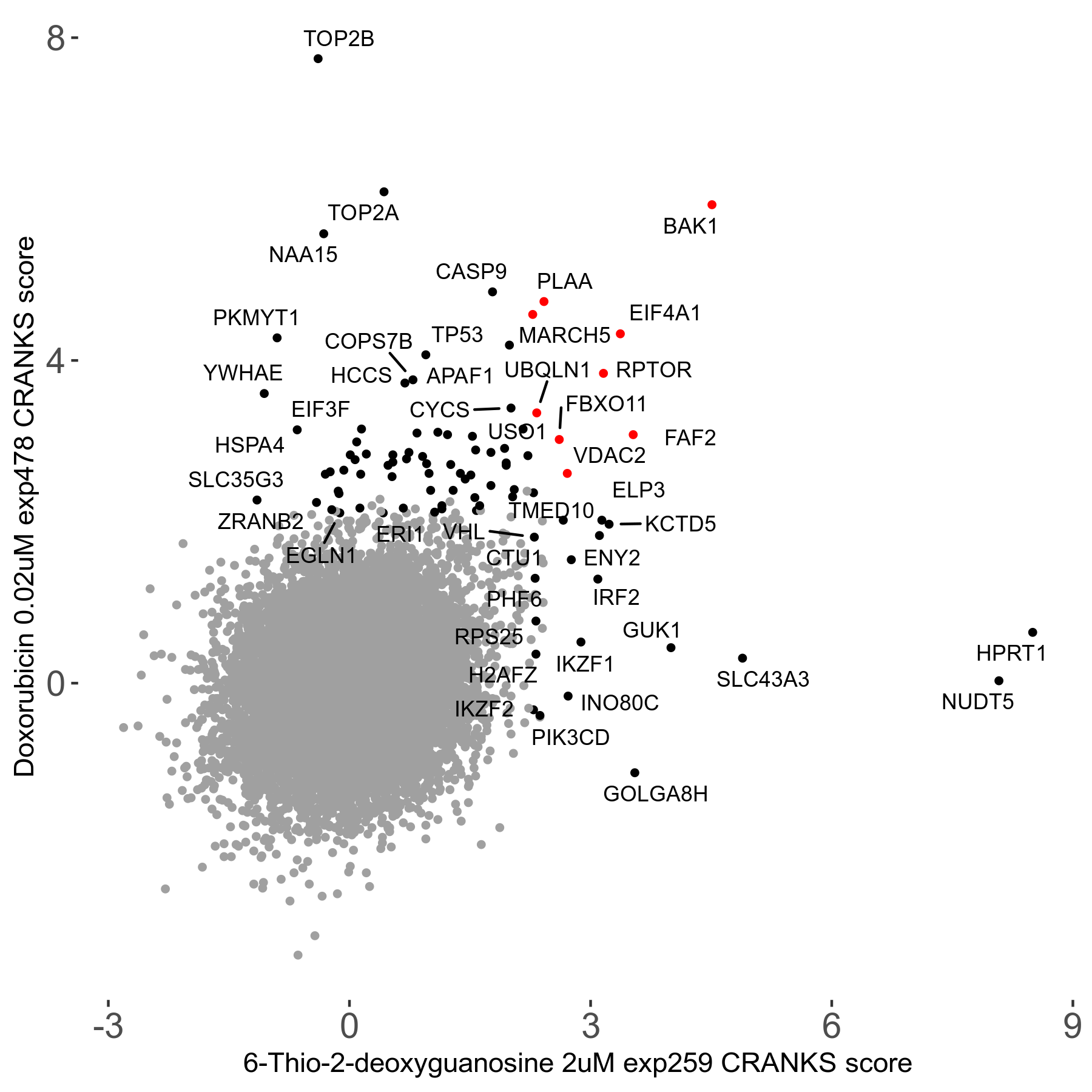
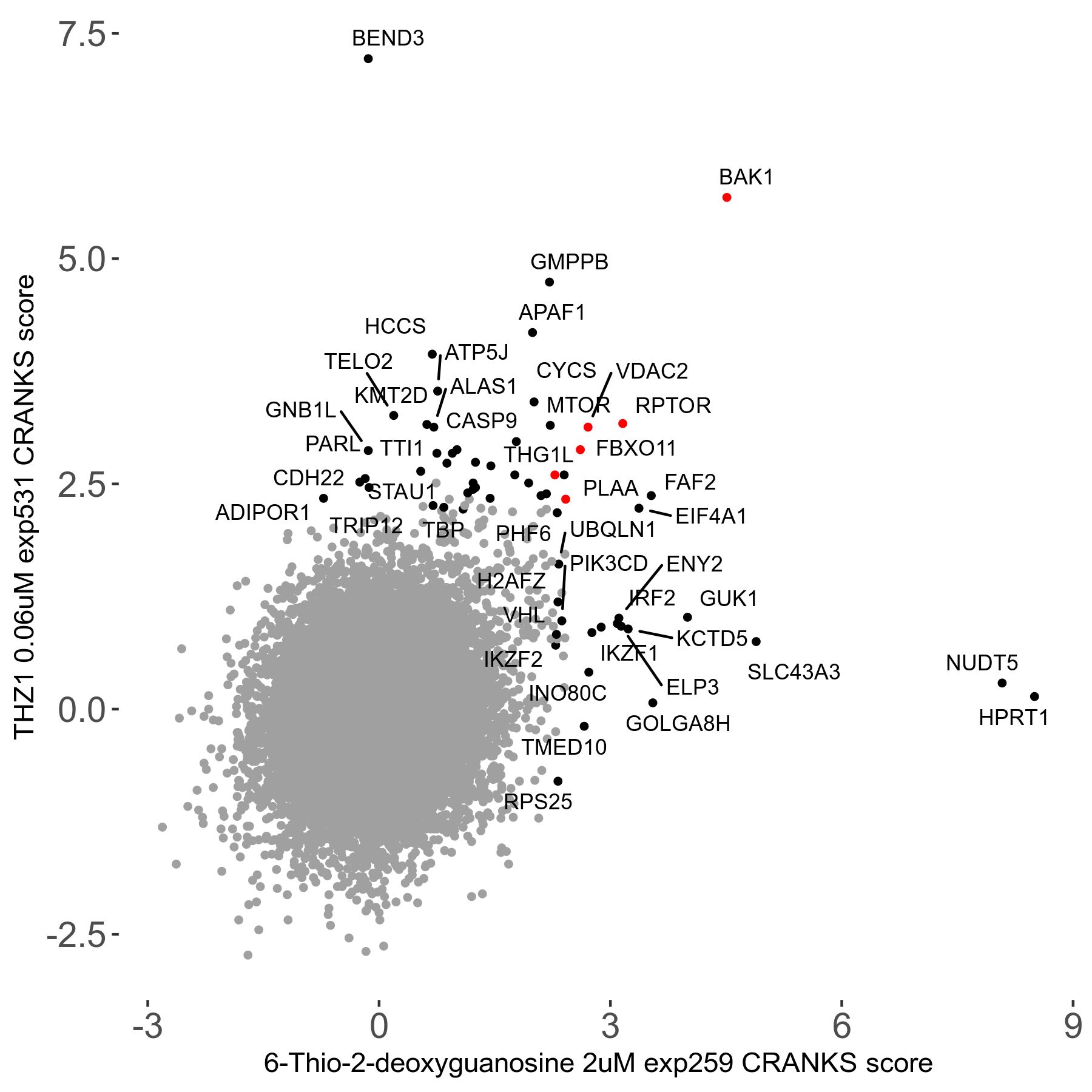
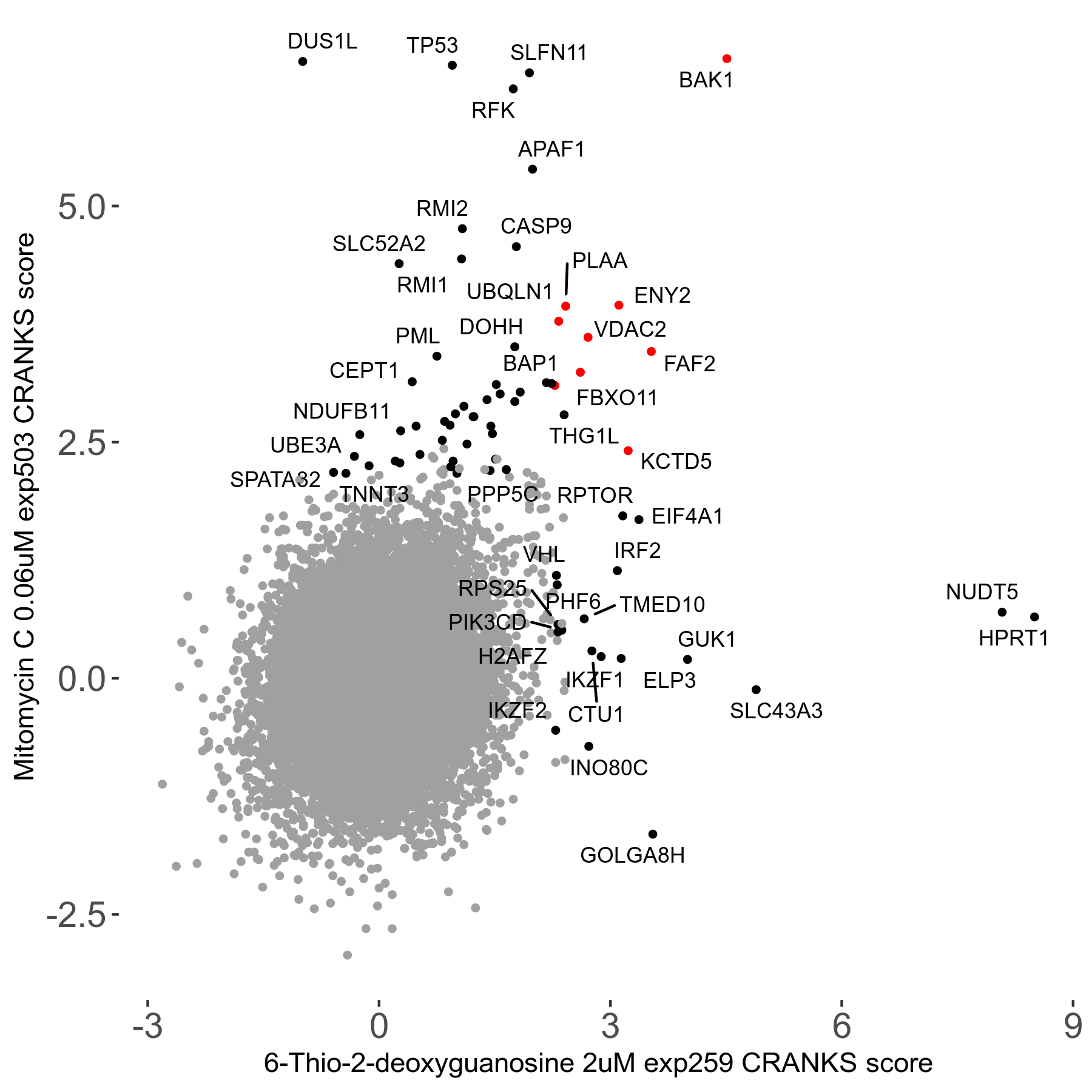
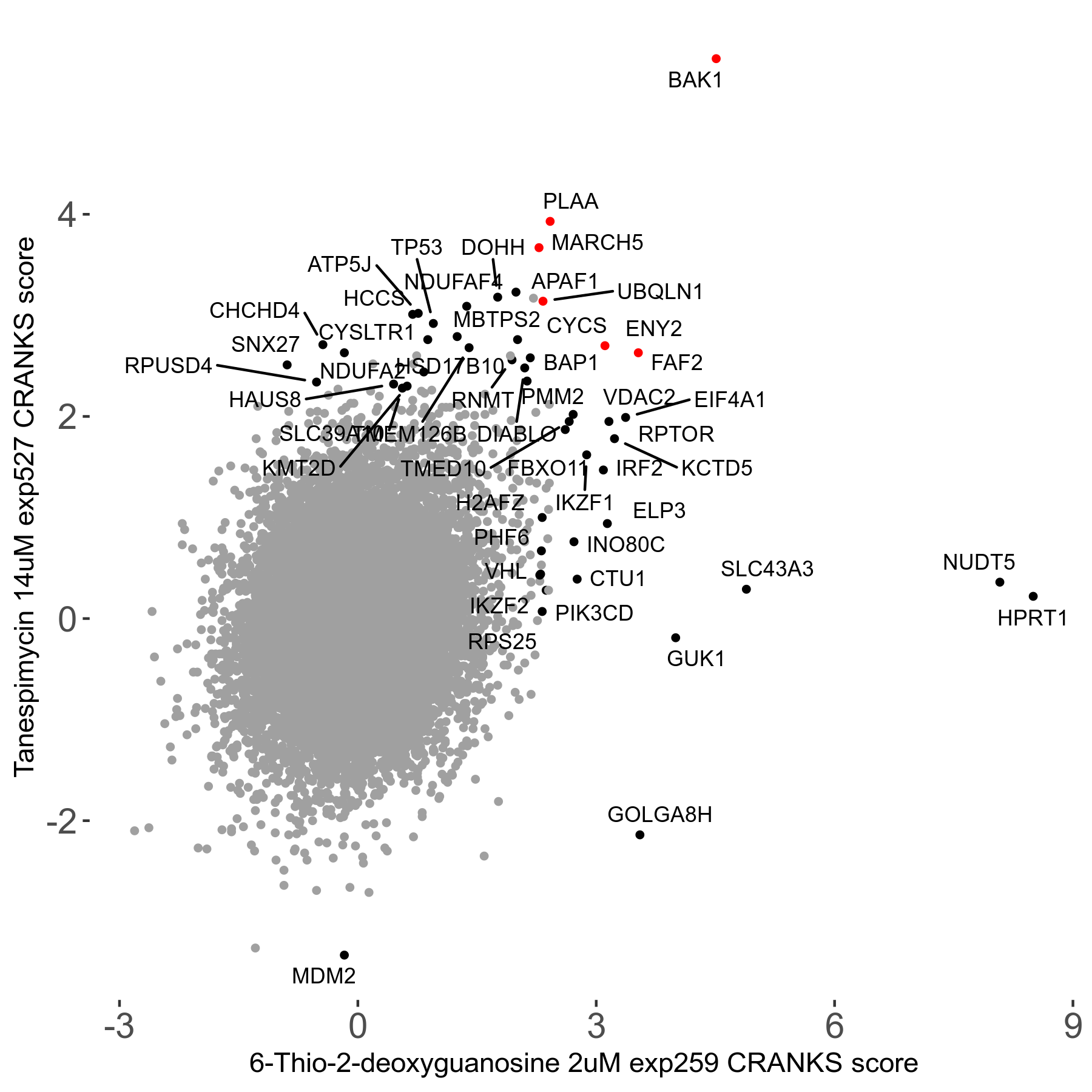
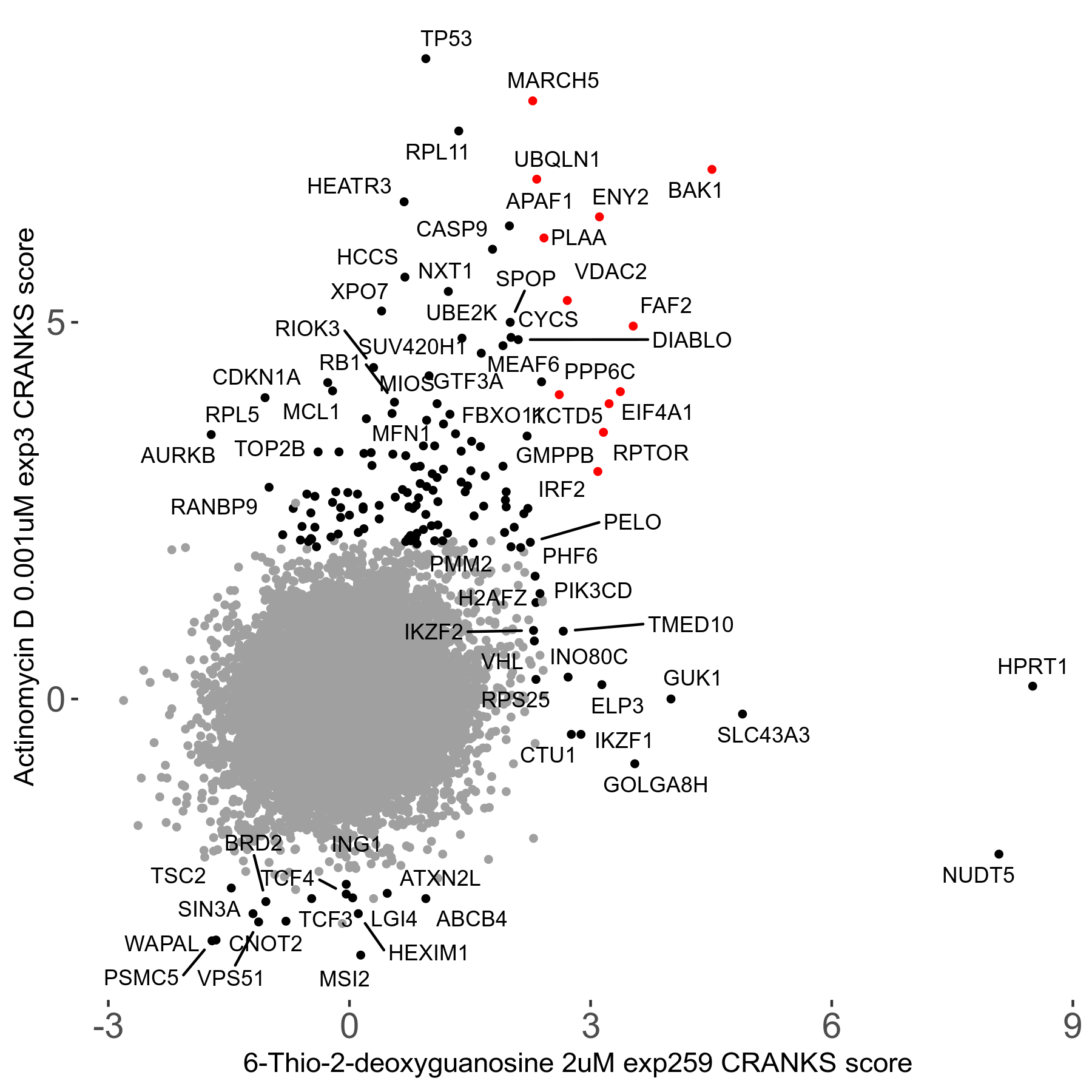
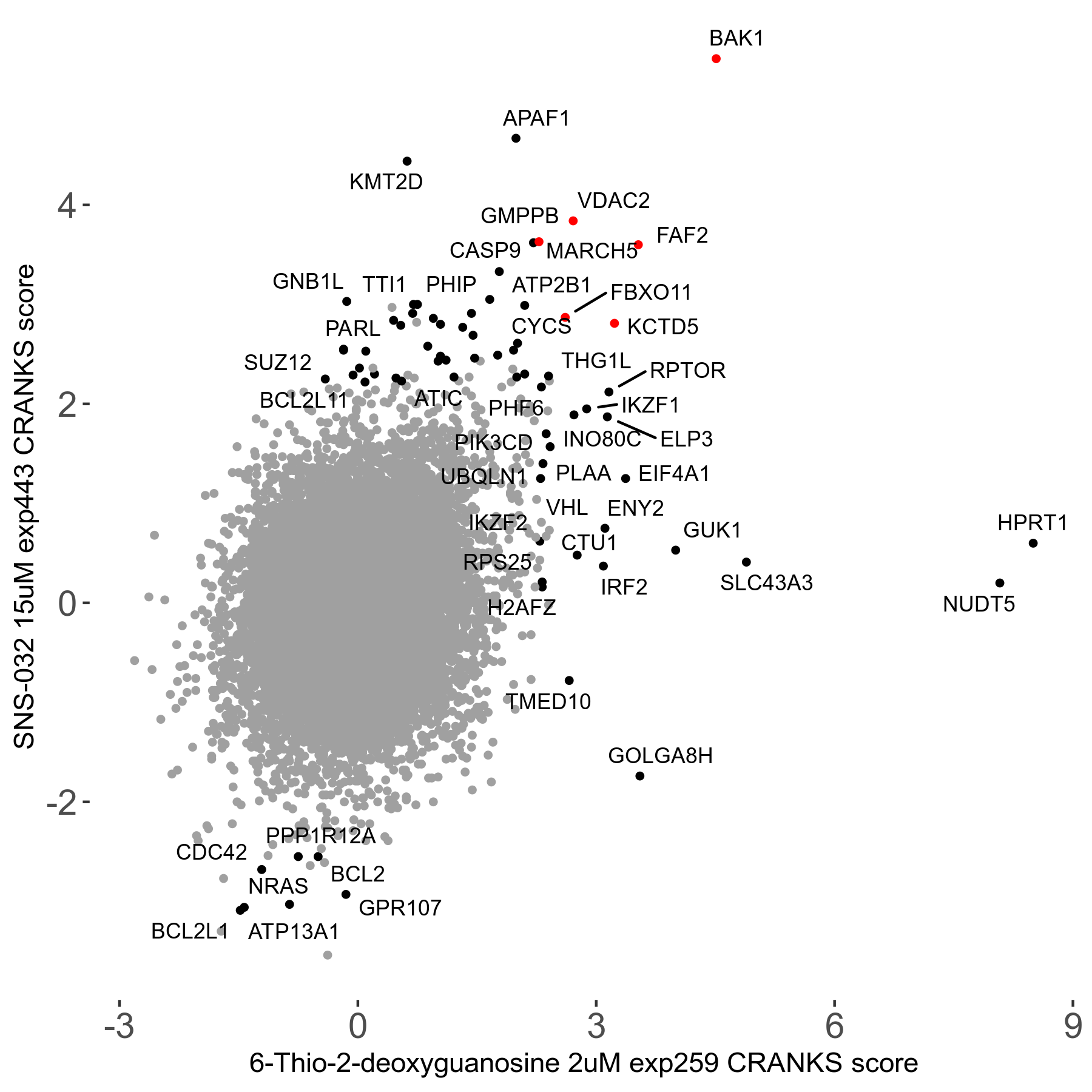
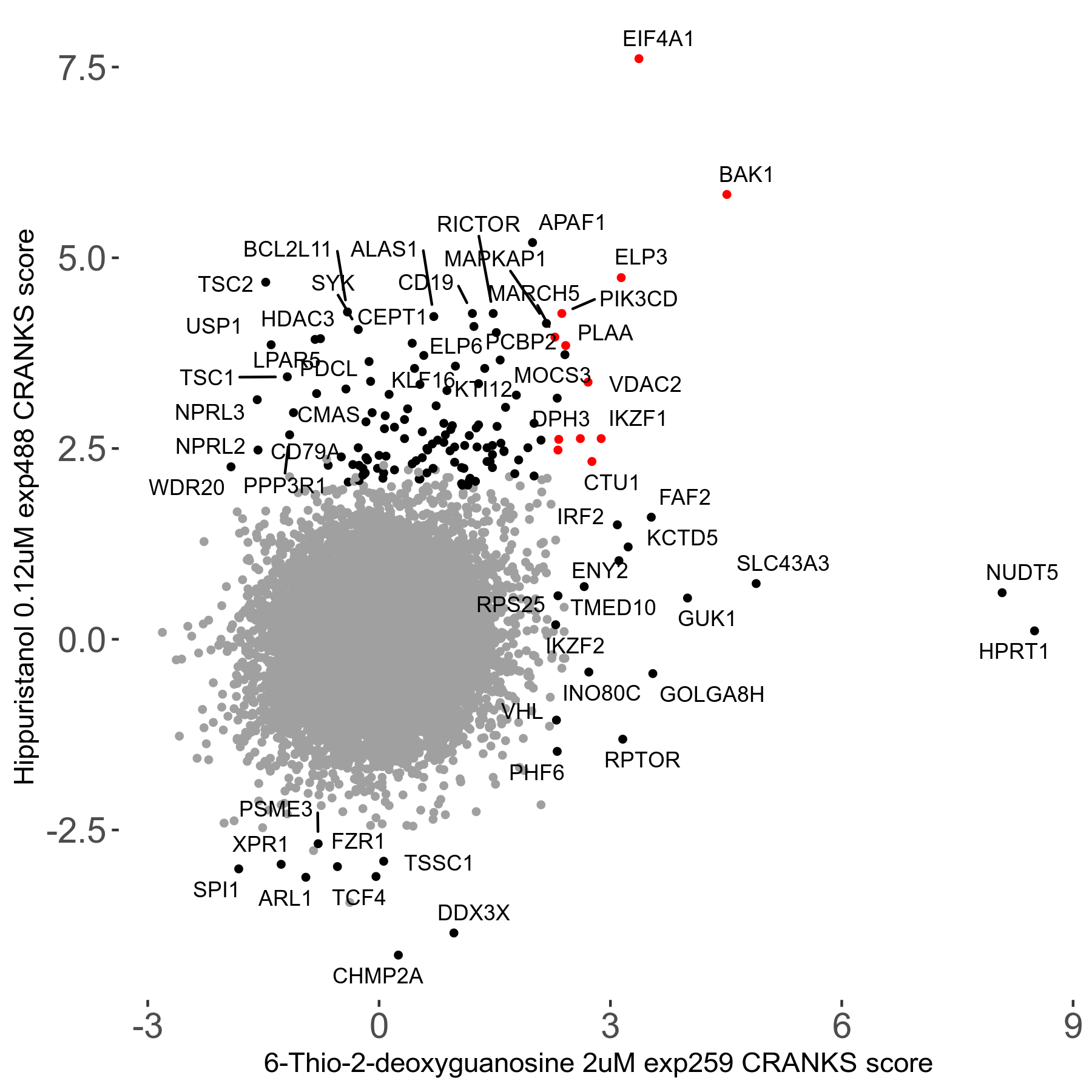
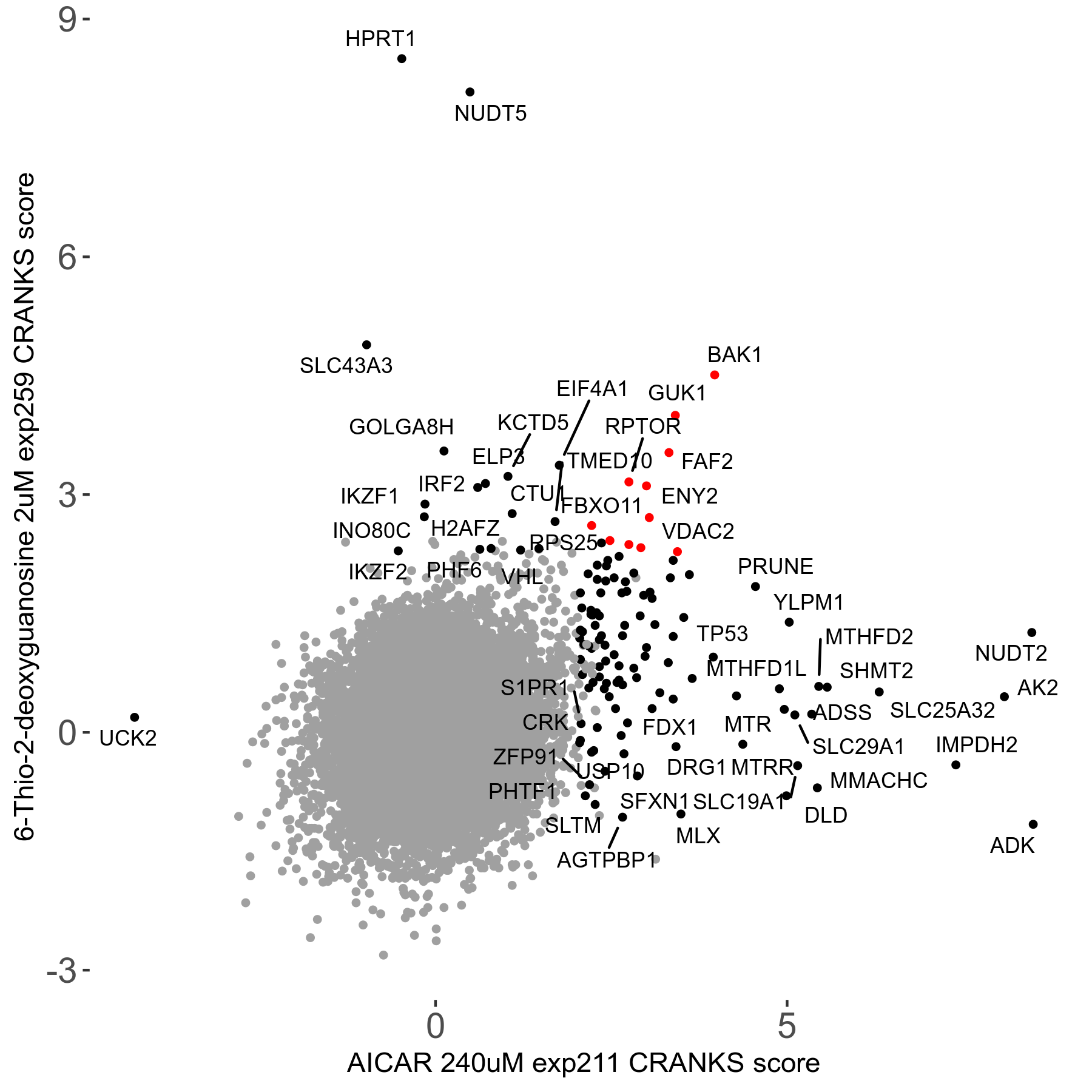
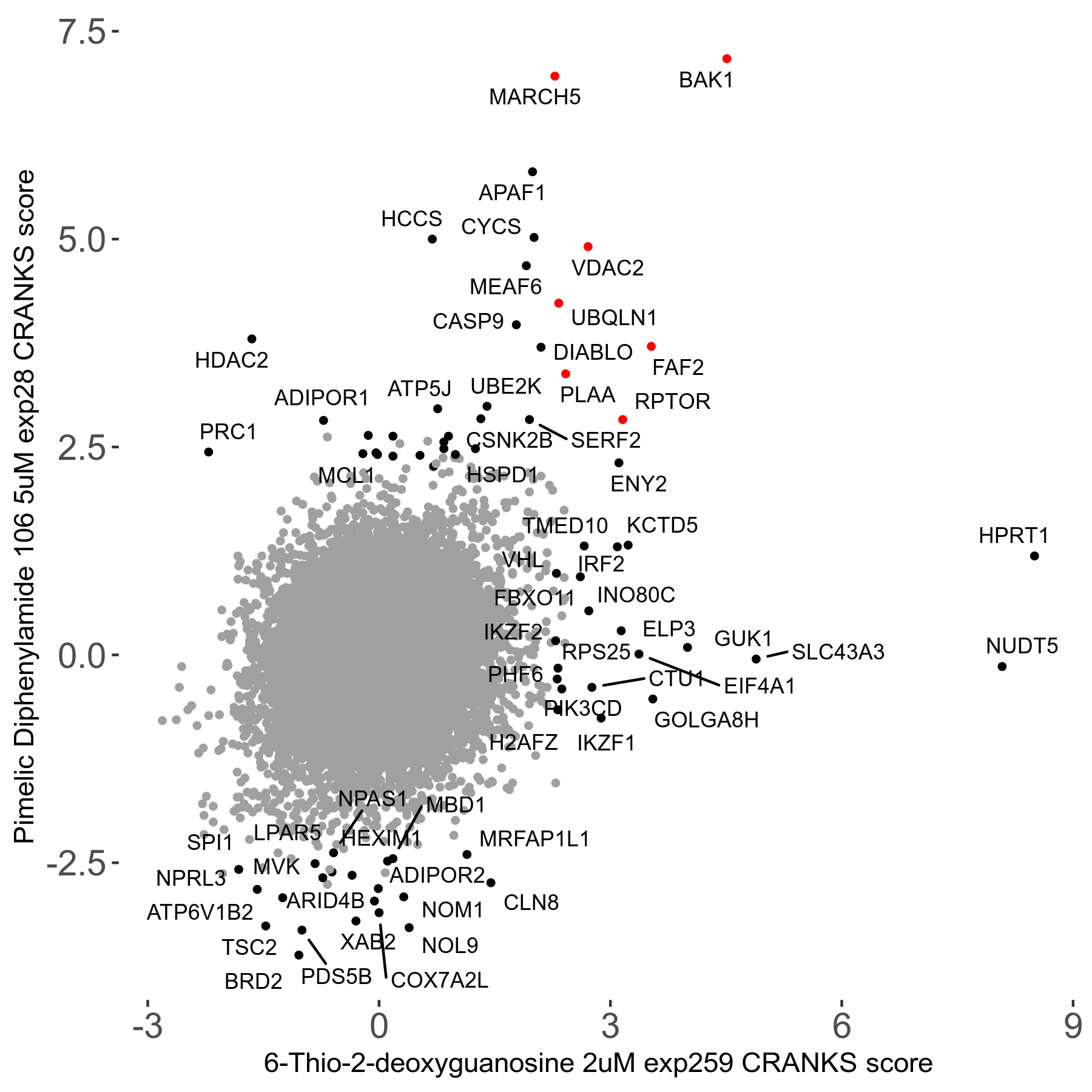
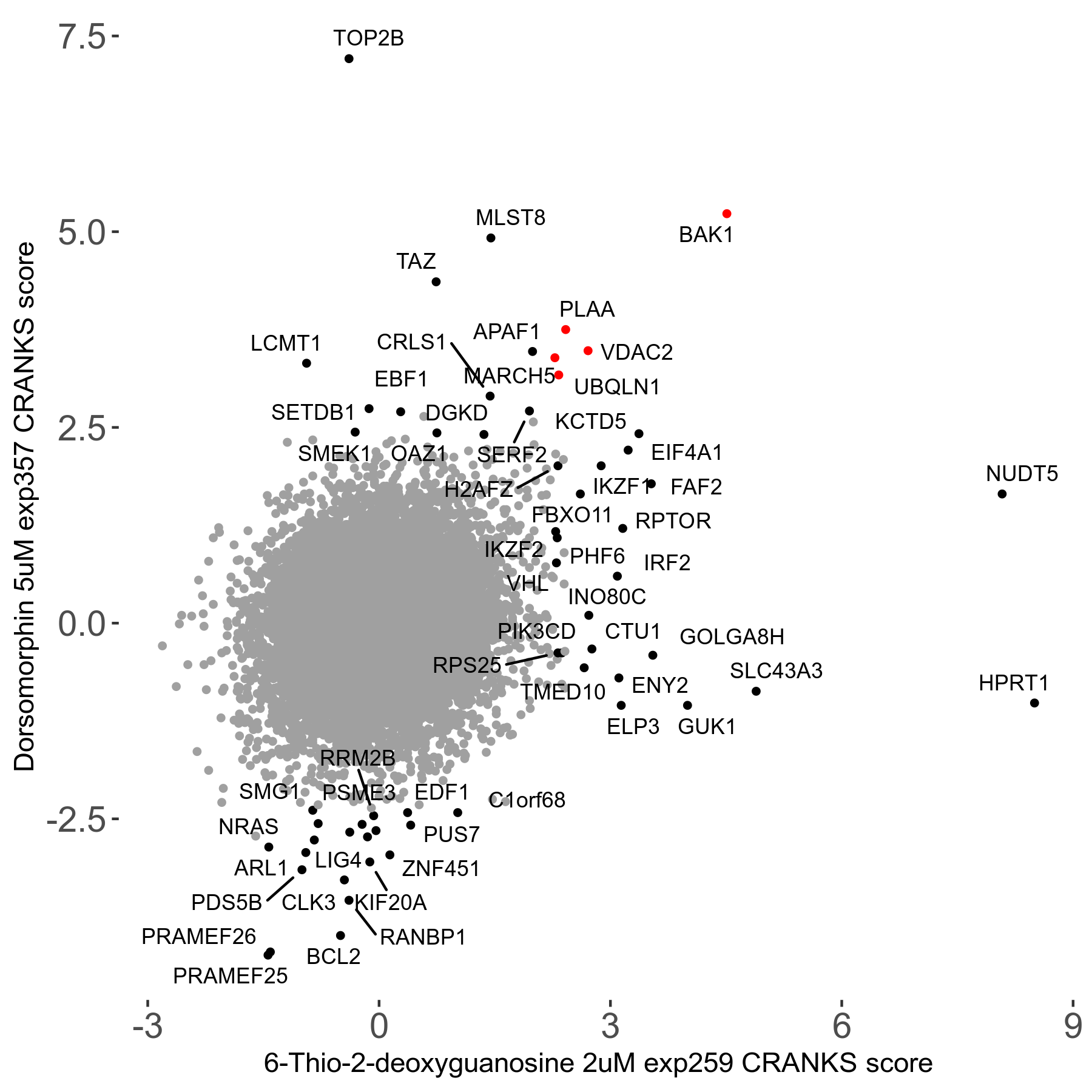
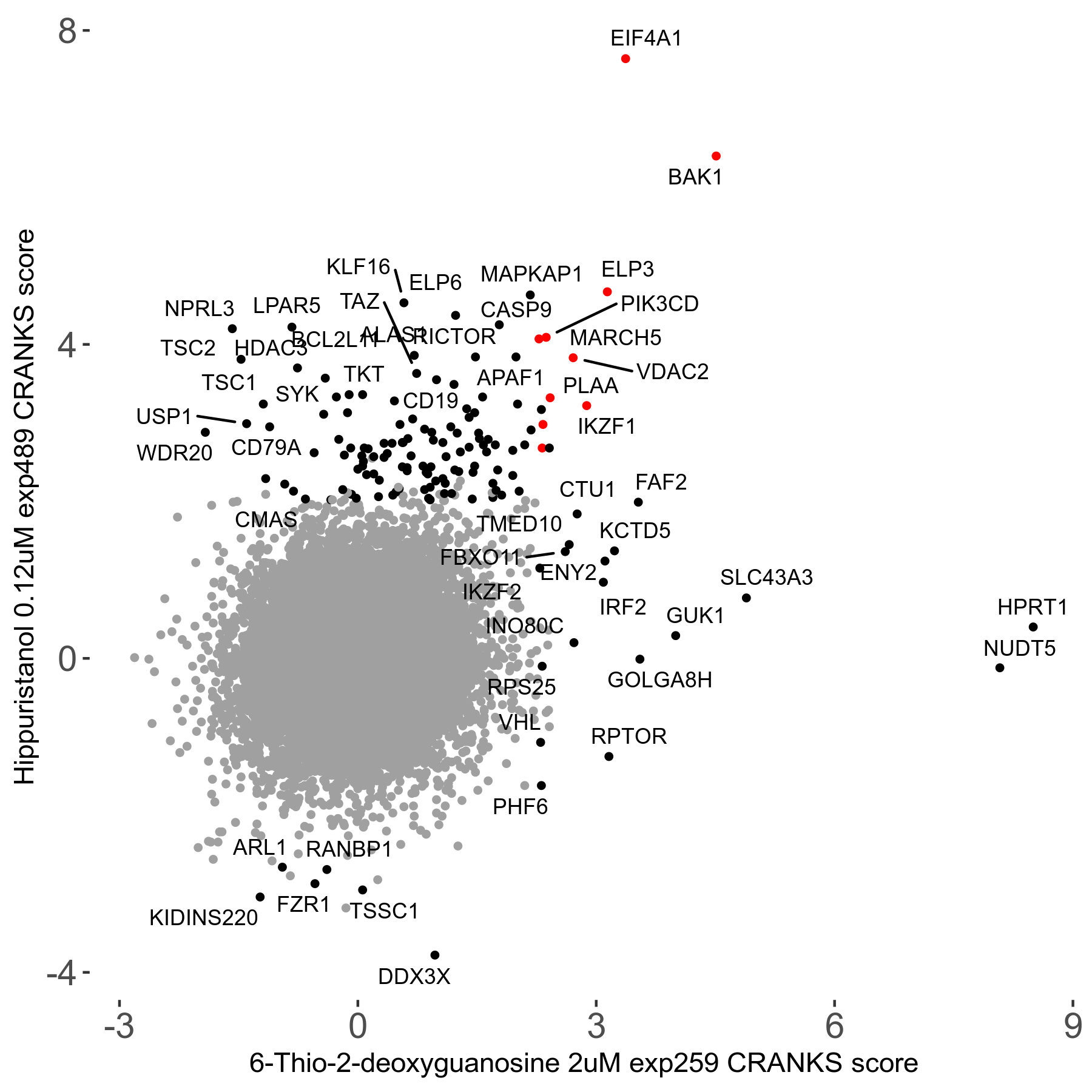
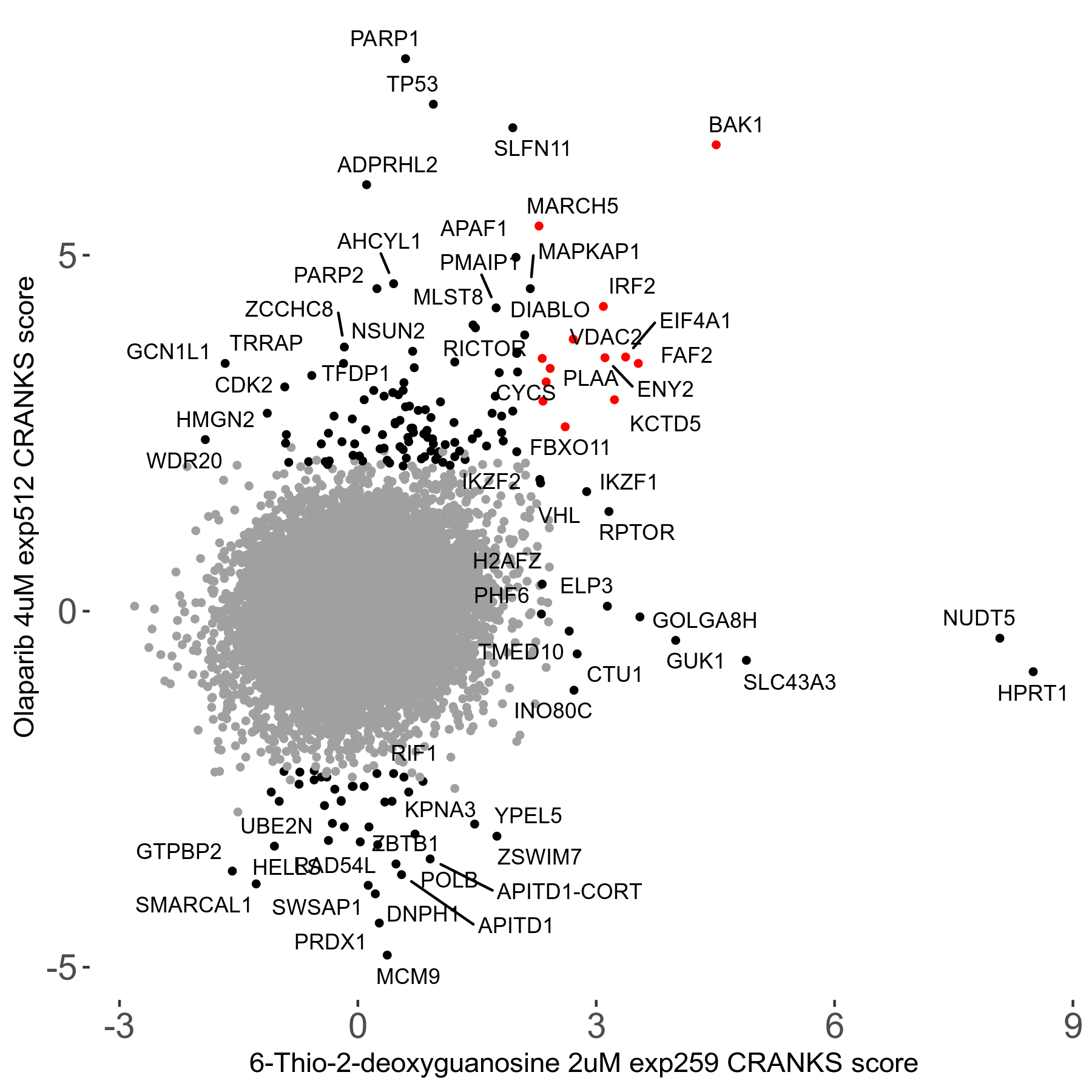
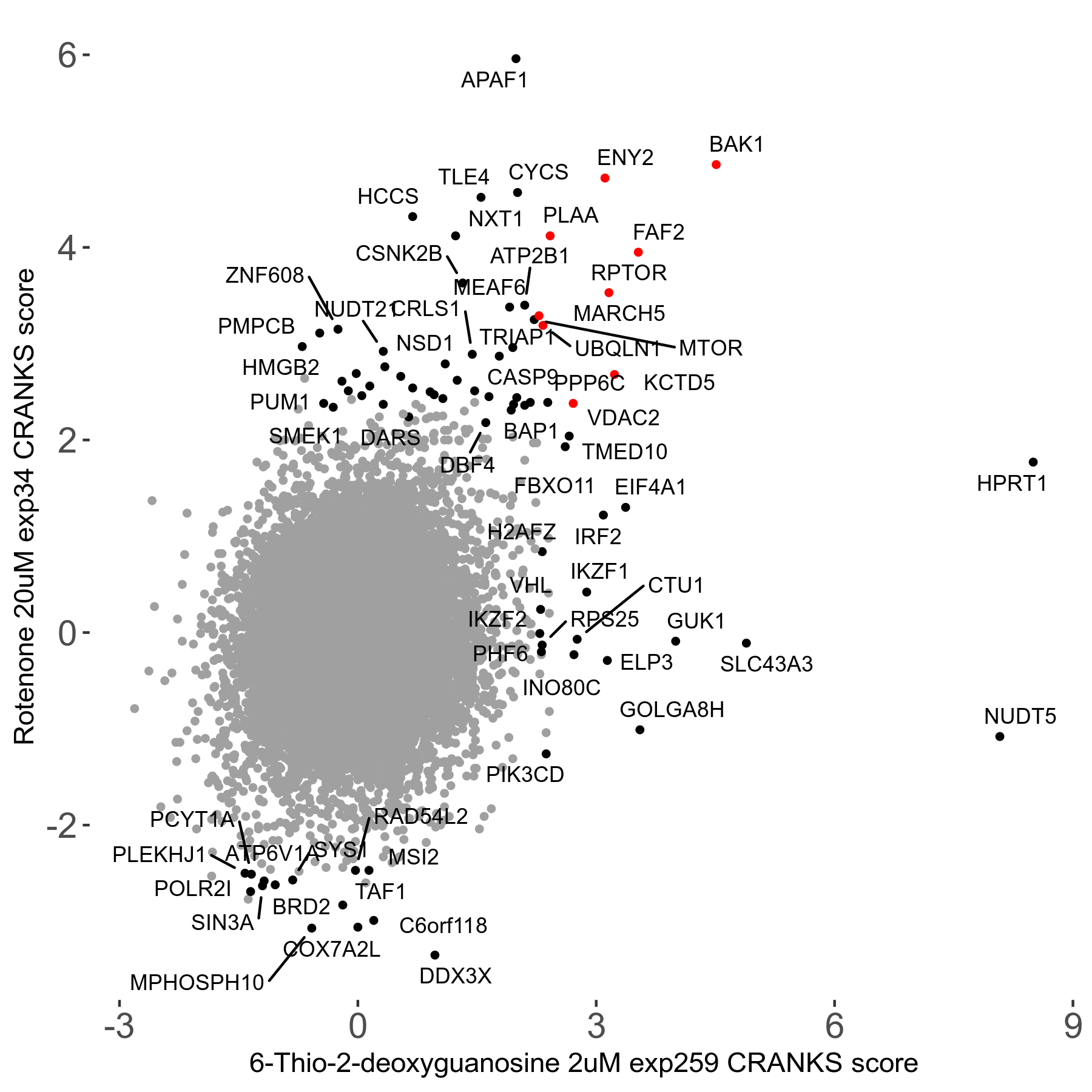
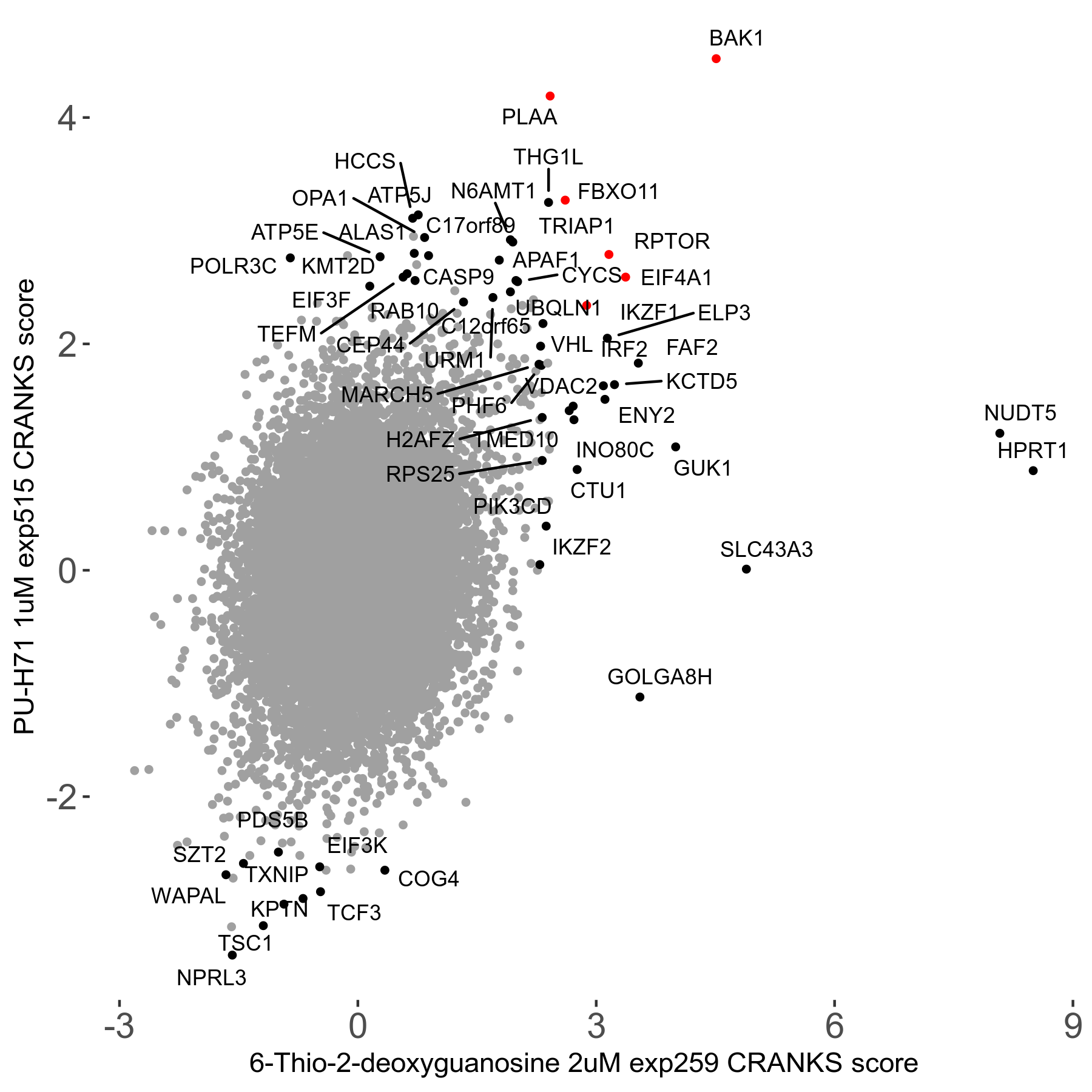
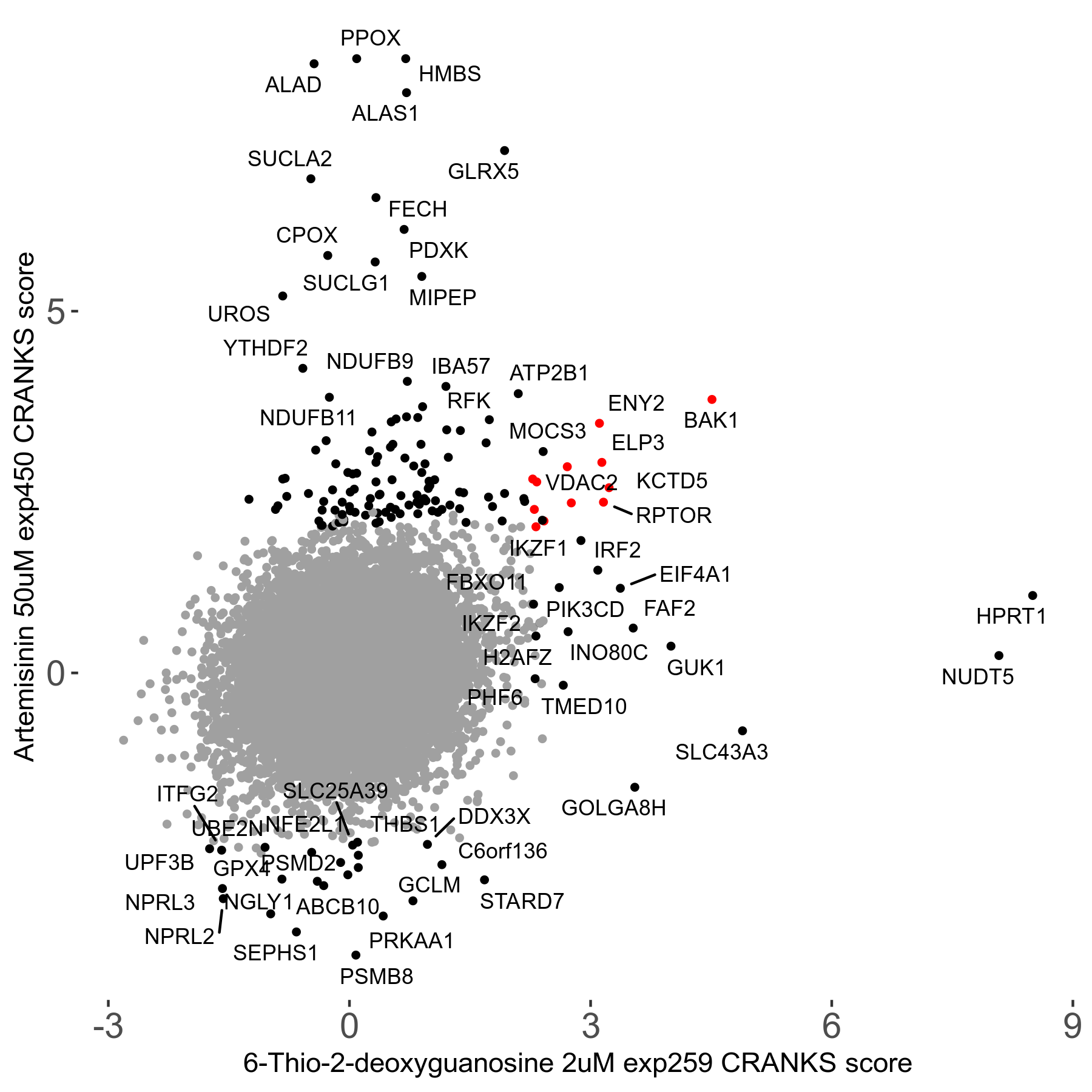
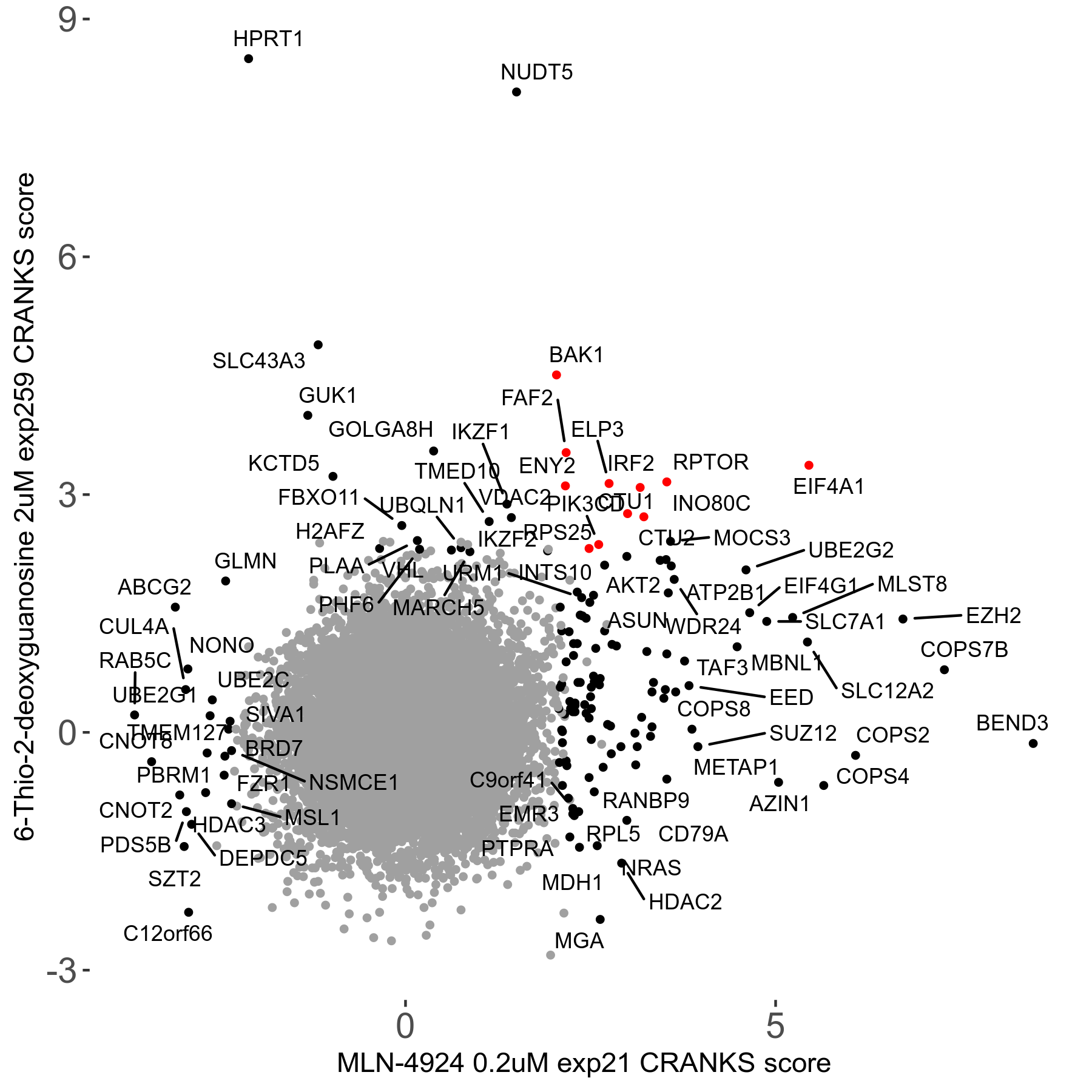
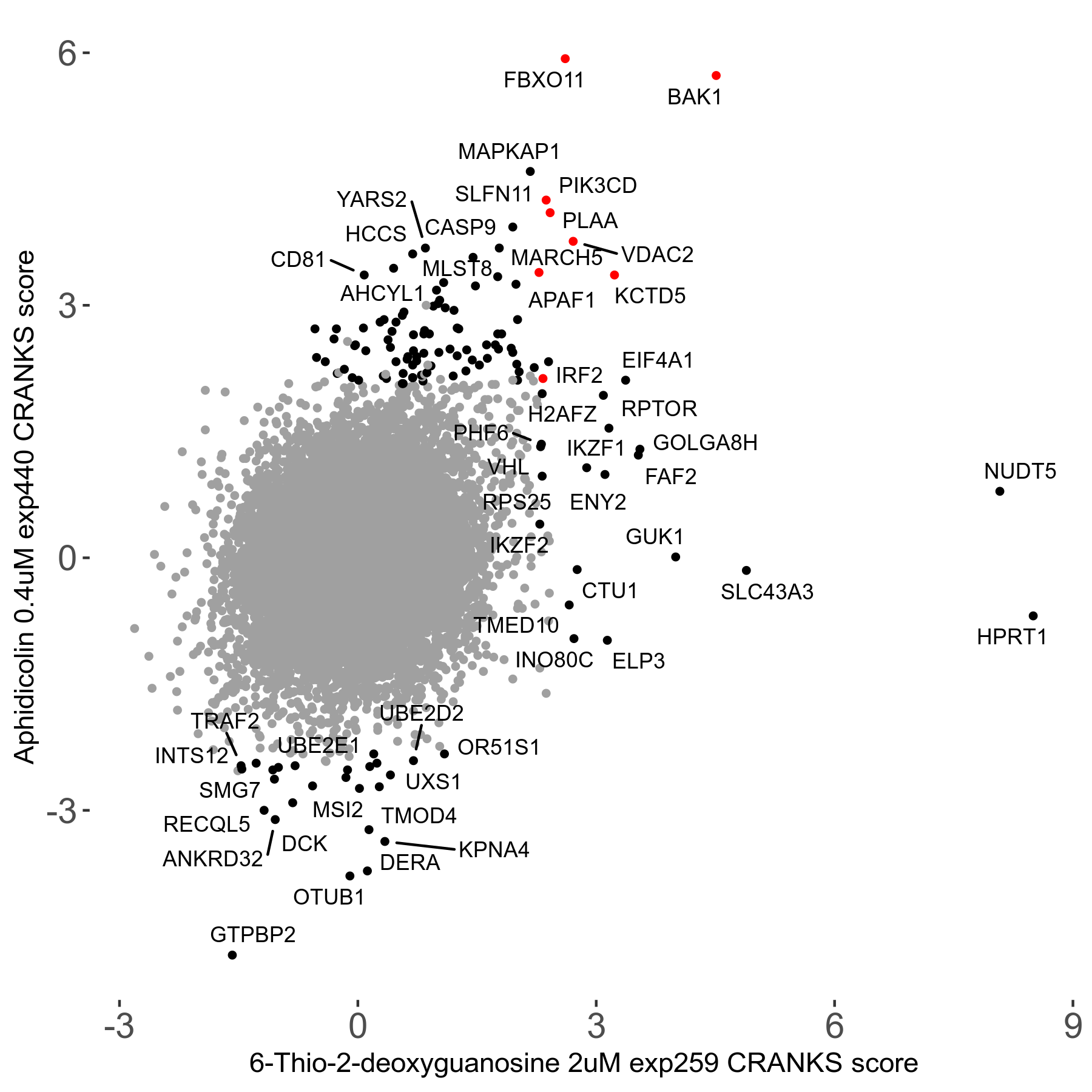
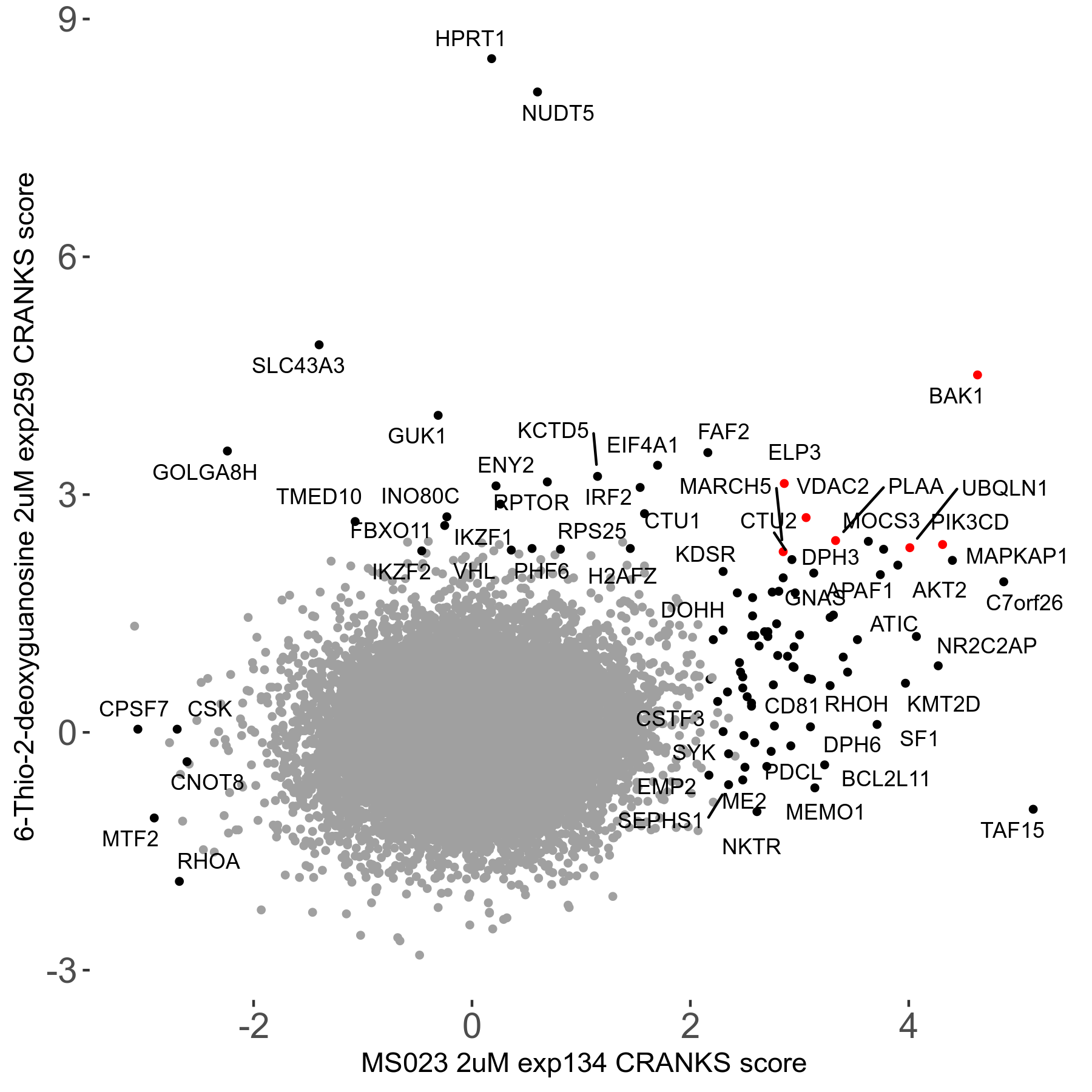
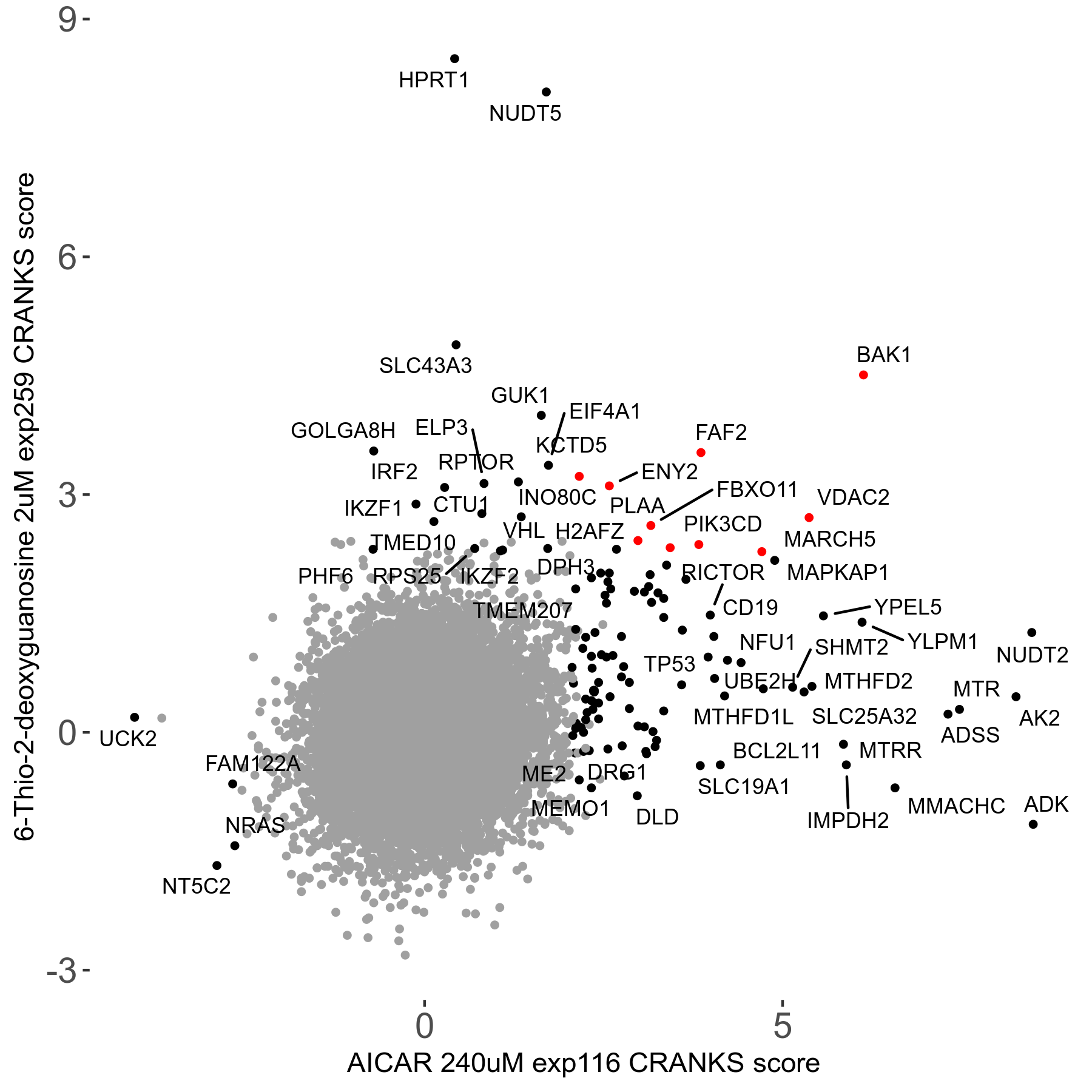
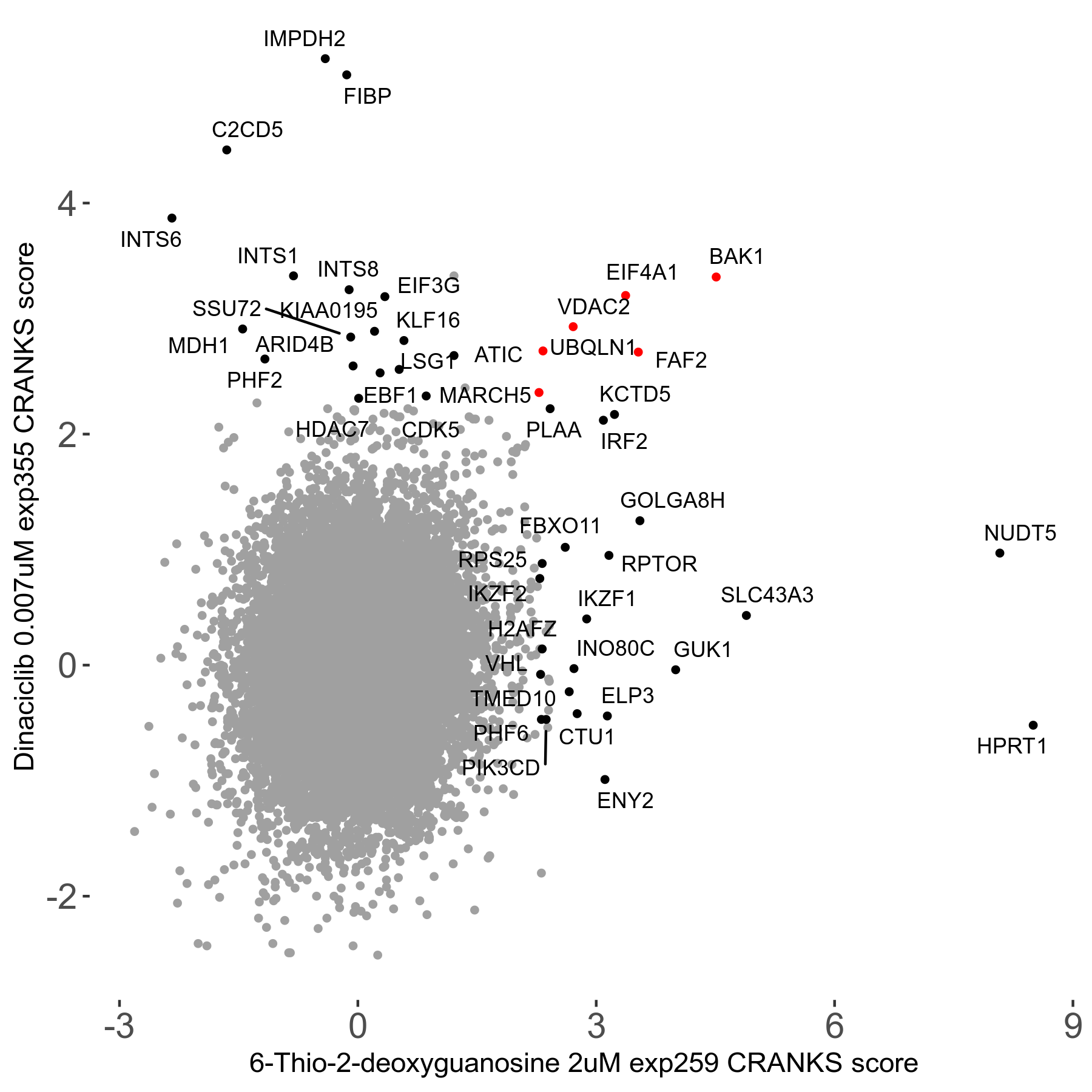
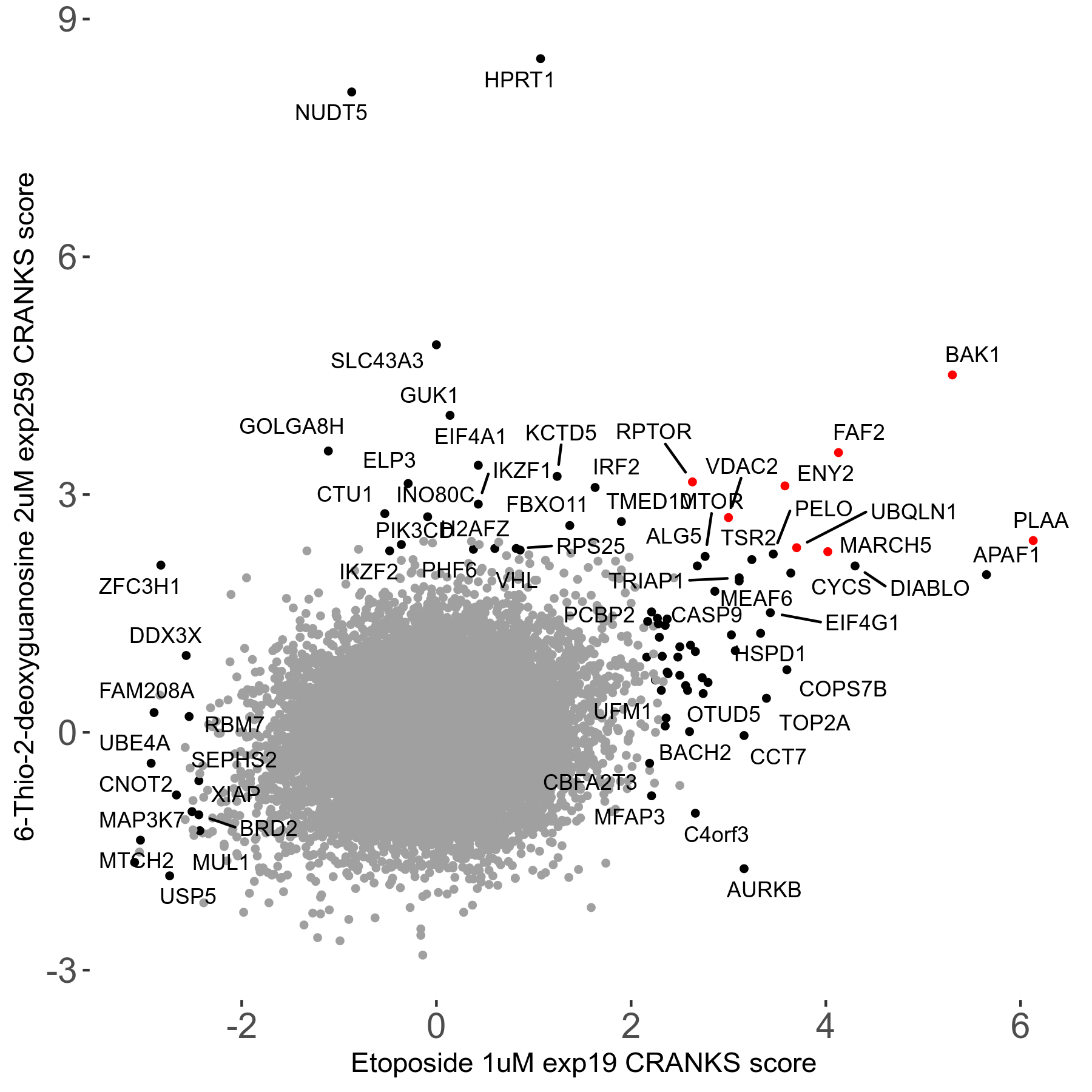
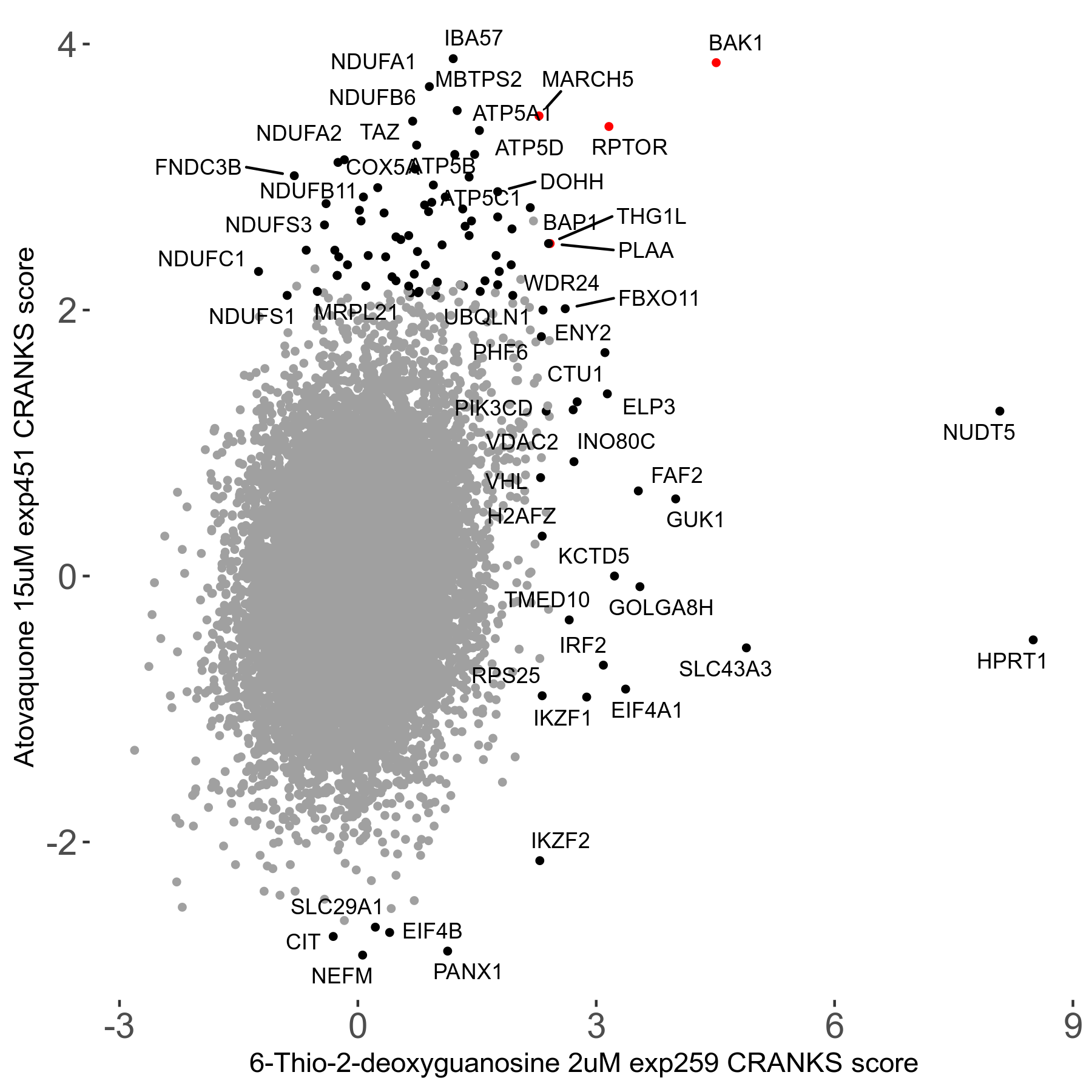
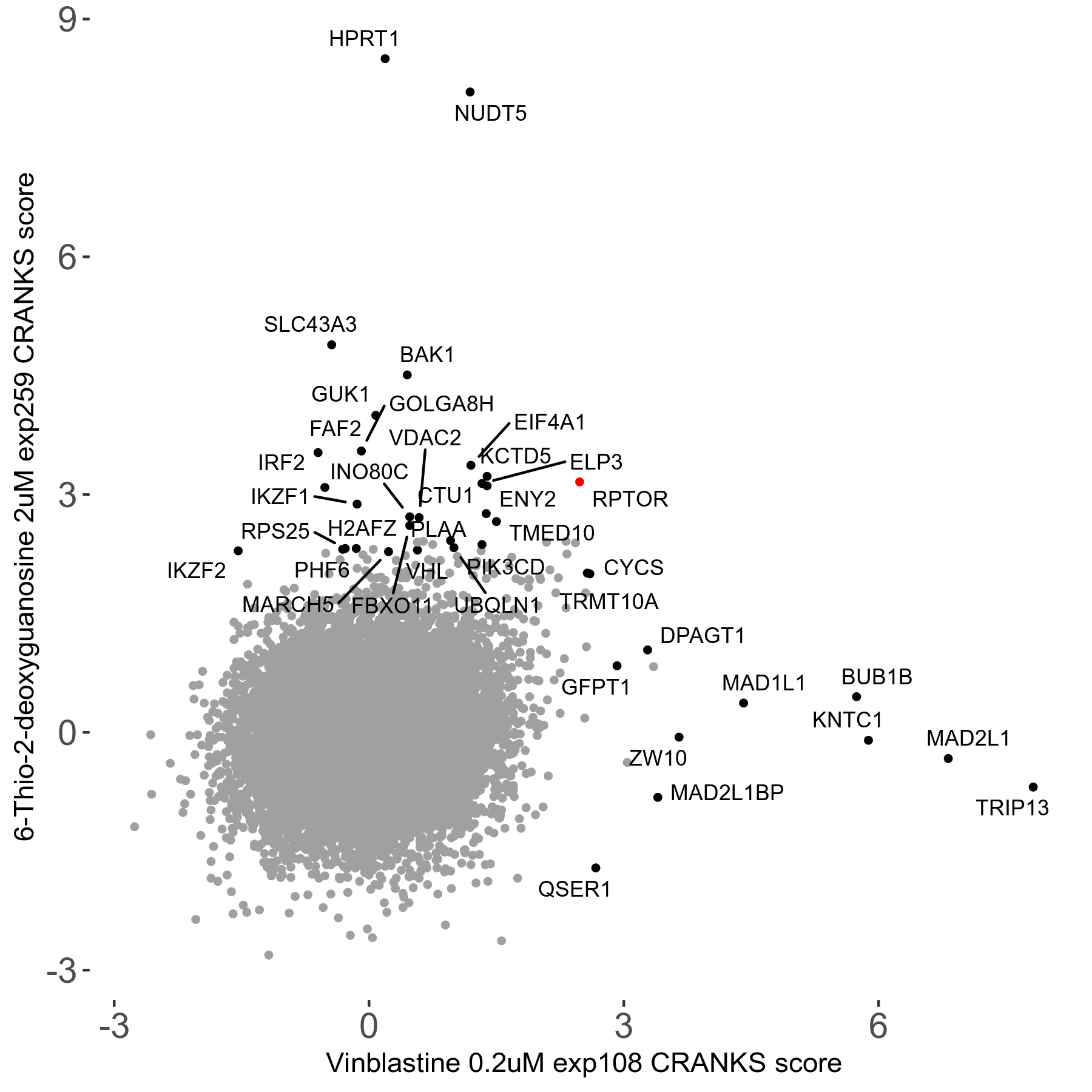
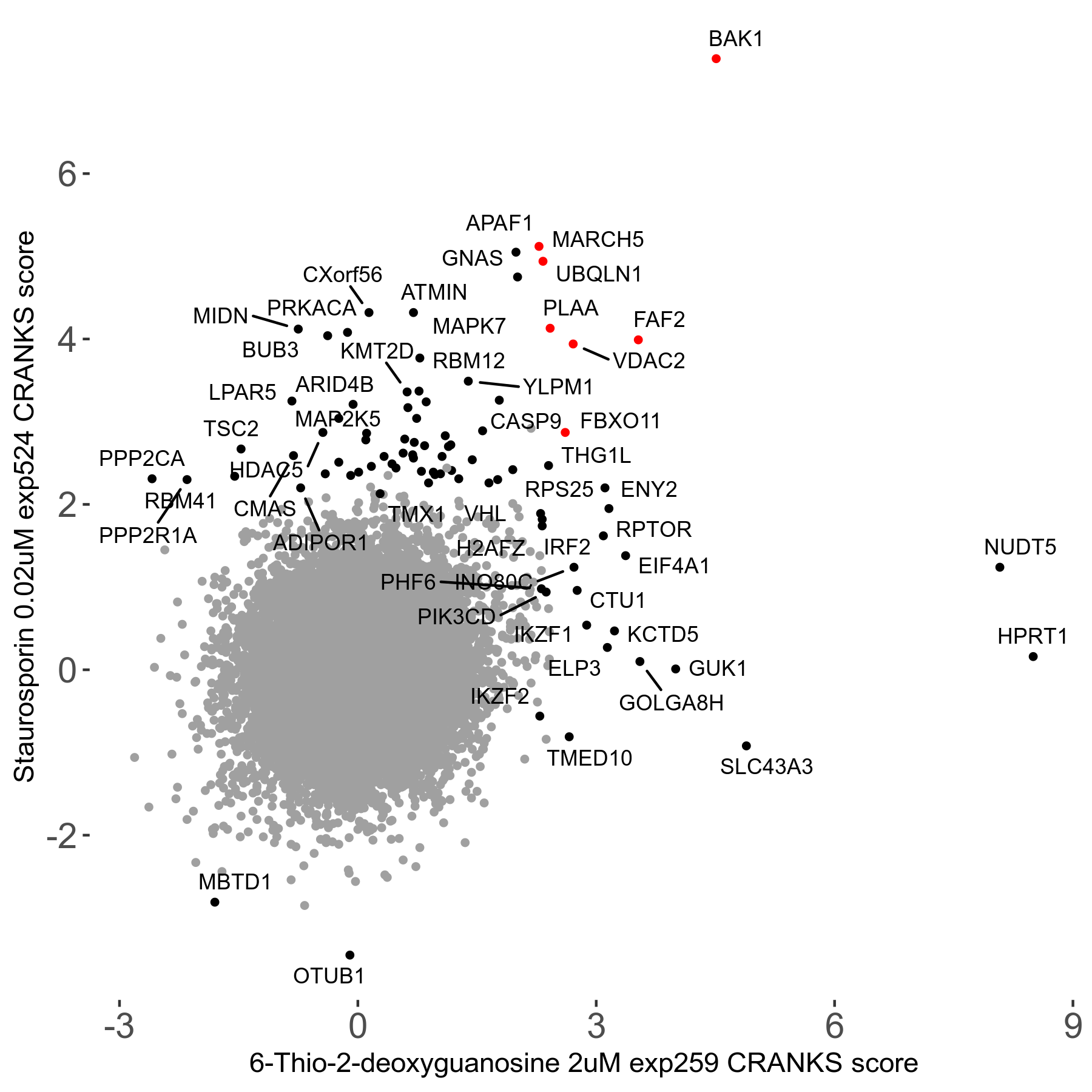
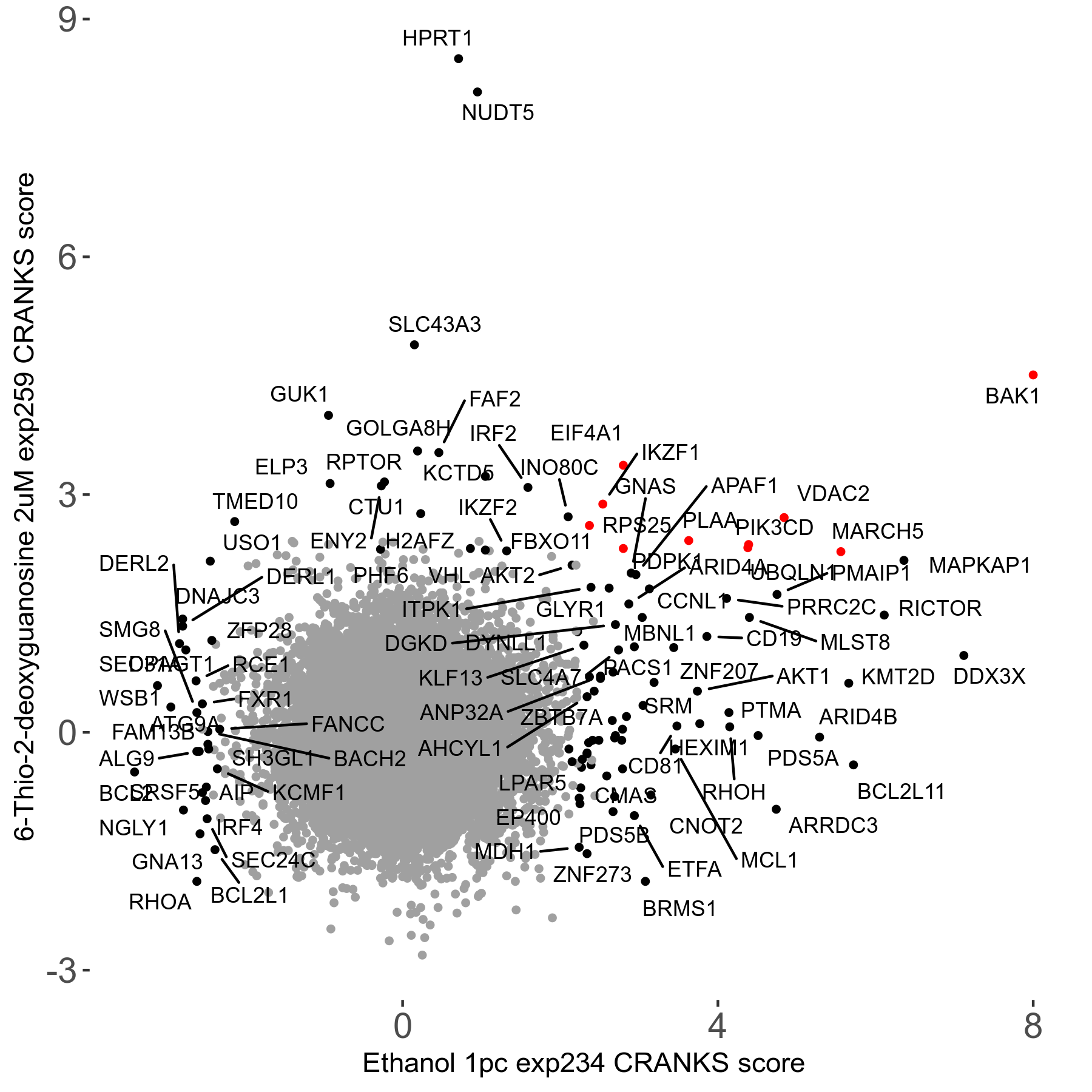
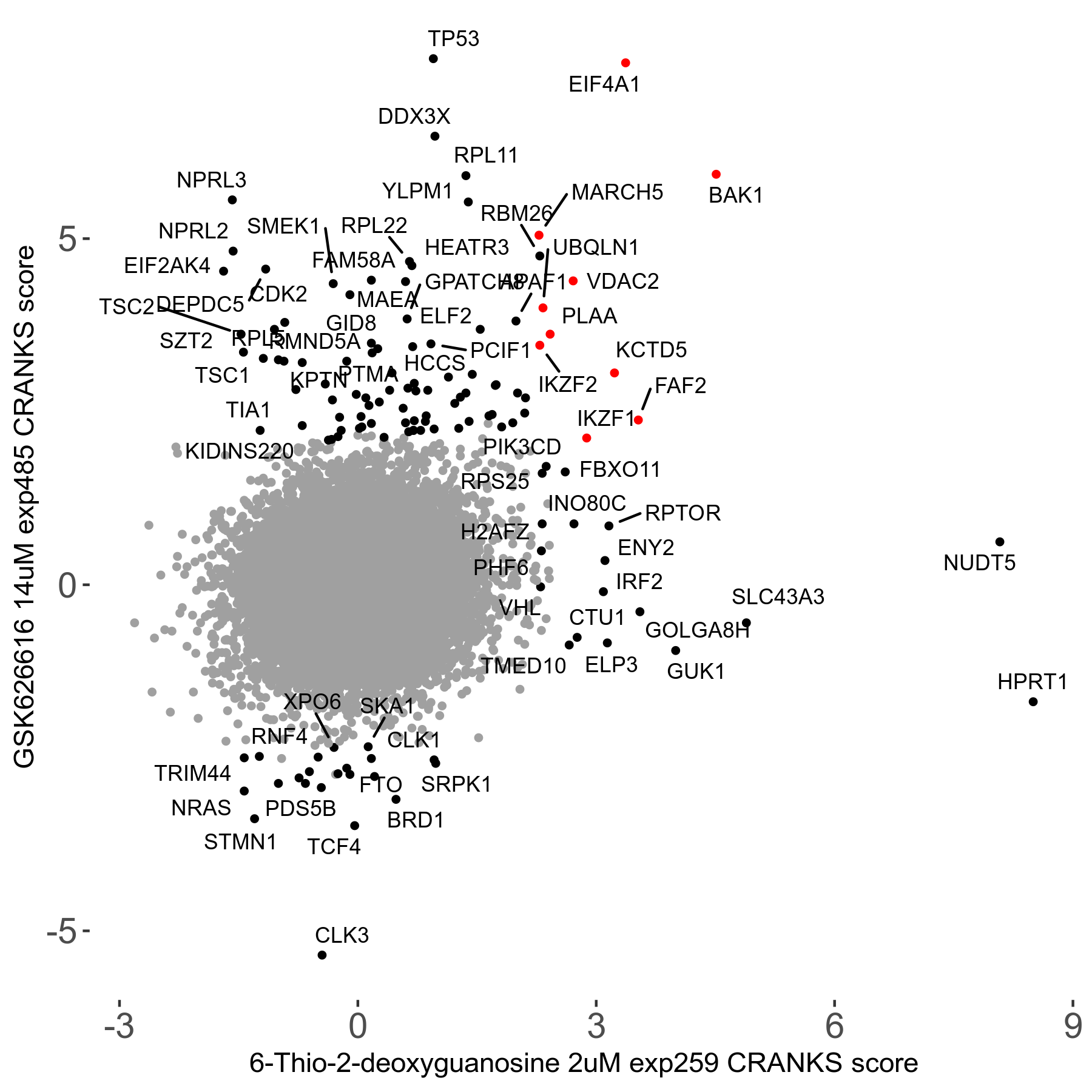
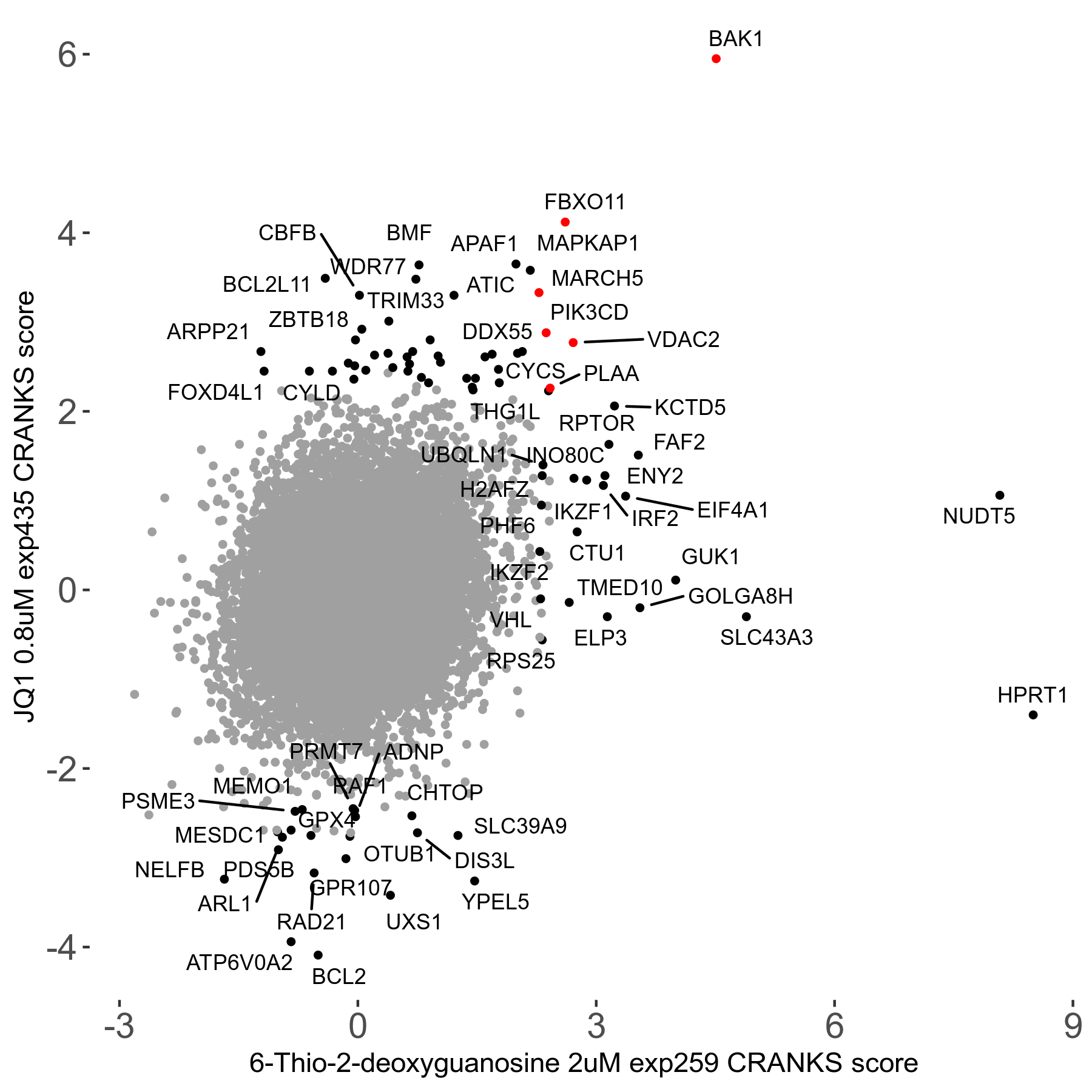
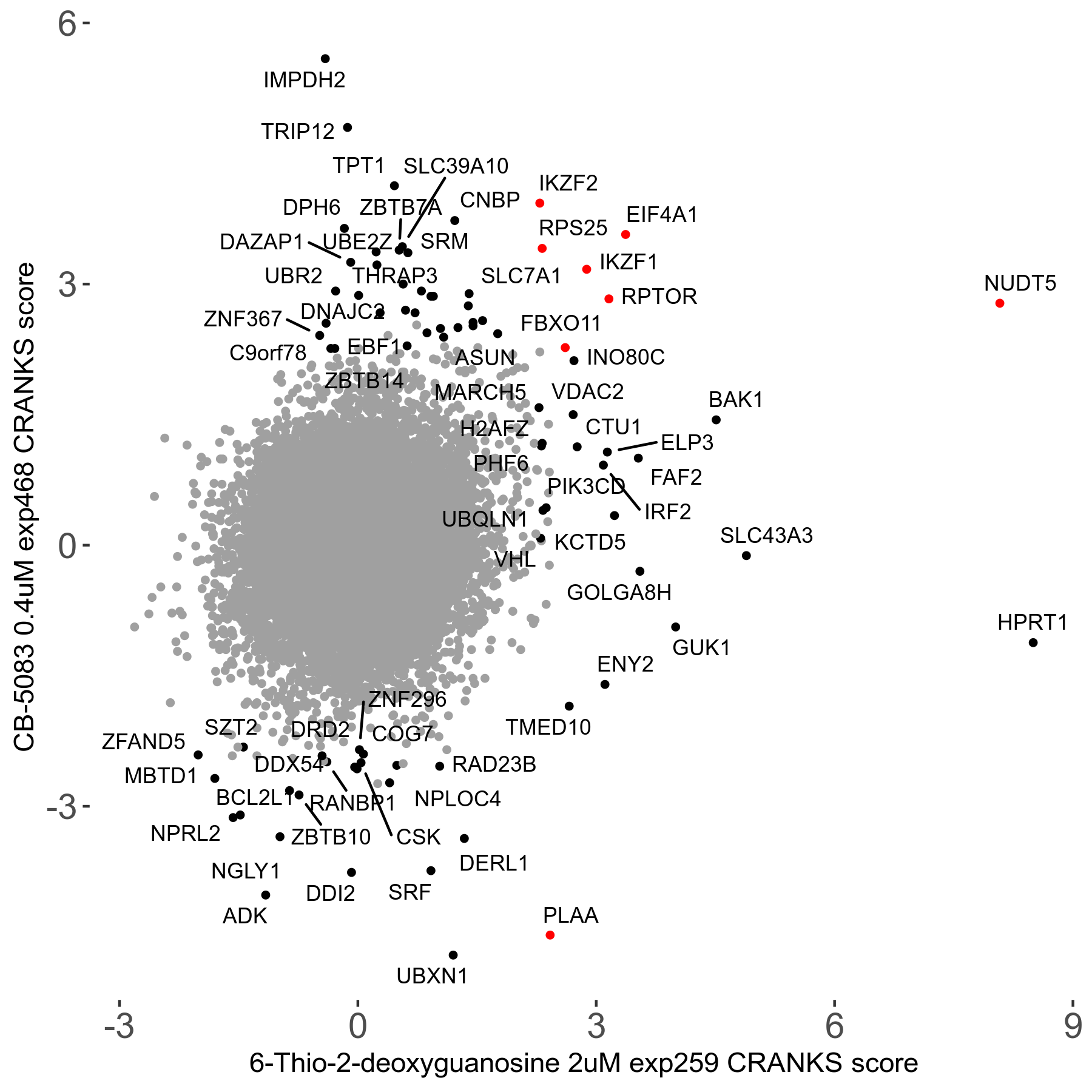
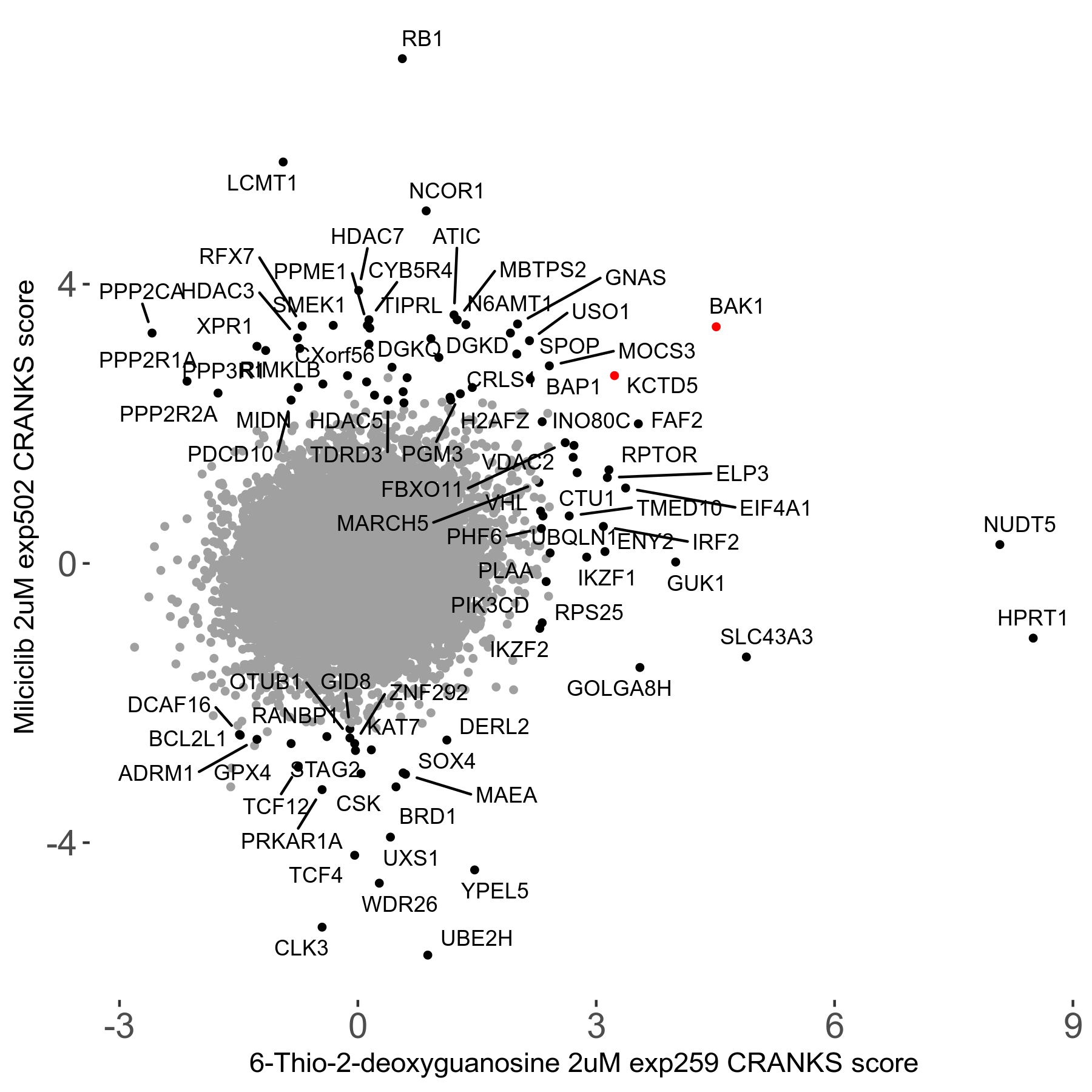
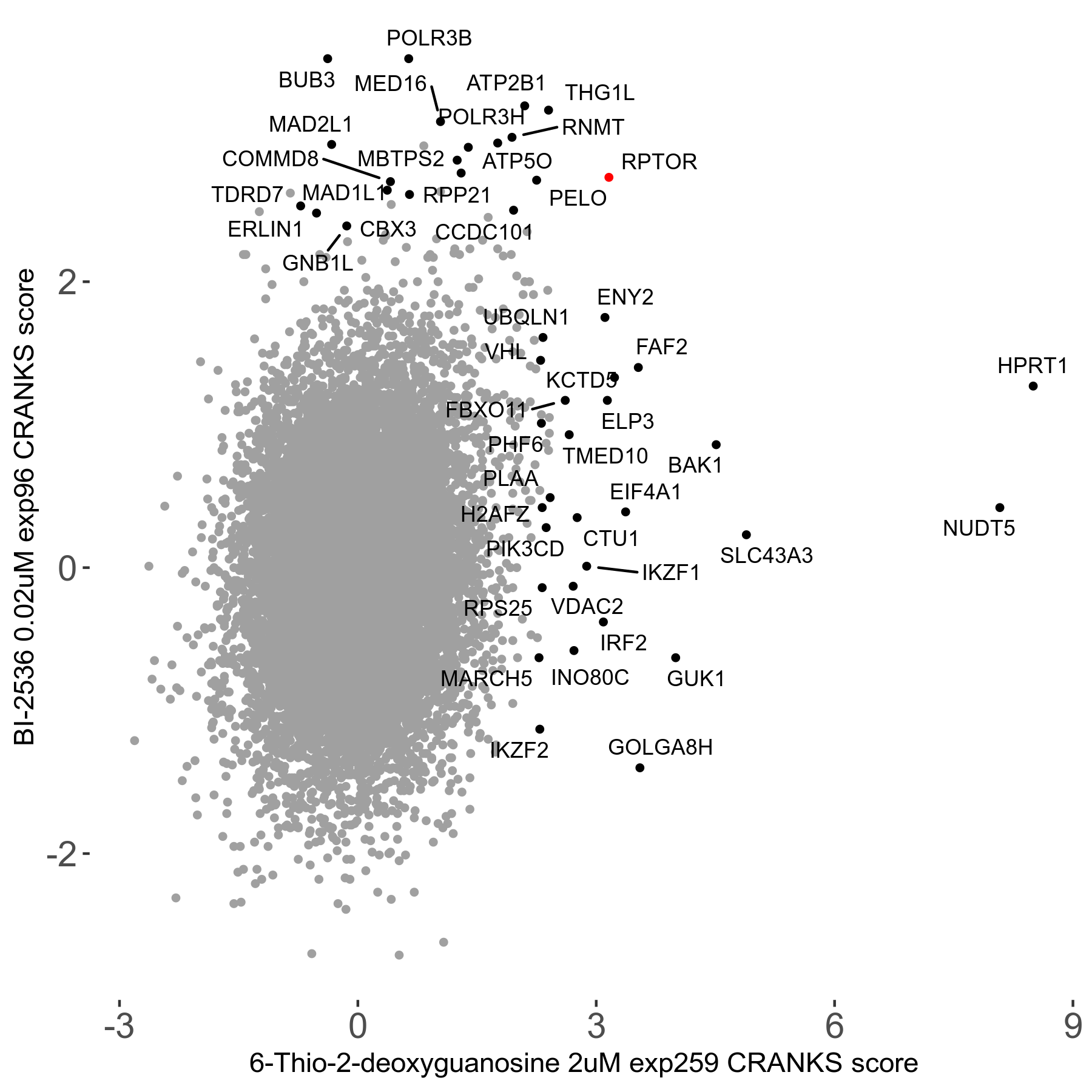
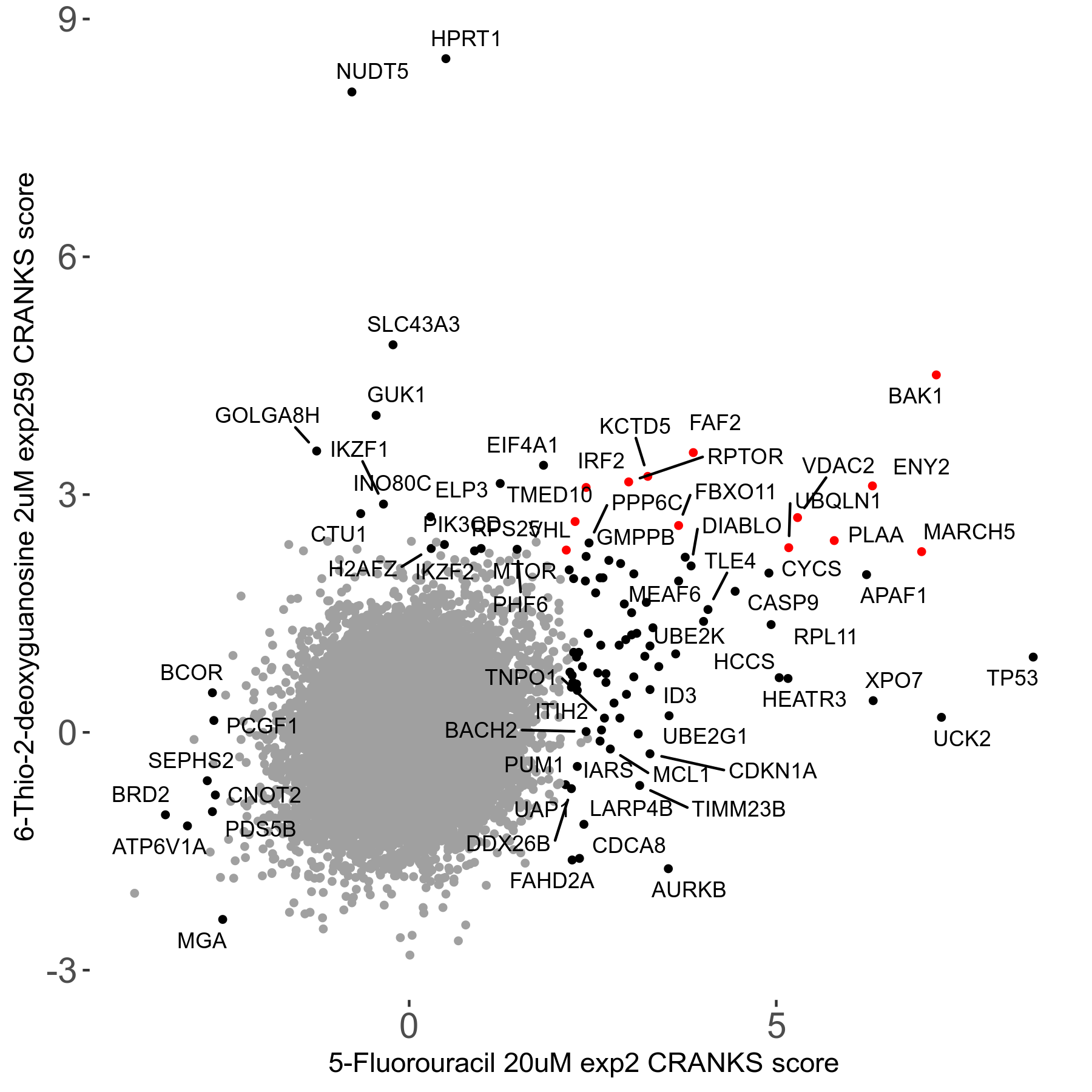
6-Thio-2-deoxyguanosine 2μM R06 exp259
Mechanism of Action
Nucleoside analog, incorporated into telomeric DNA by telomerase, impairs proliferation of telomerase-positive cells
- Class / Subclass 1: Metabolism / Antimetabolite
- Class / Subclass 2: DNA Damage, Repair and Replication / Helix-Distorting Adduct
Technical Notes
Compound References
- PubChem Name: 2'-Deoxythioguanosine
- Synonyms: 6-thio-dG; β-TGdR; 6-thio-dG;β-TGdR
- CAS #: 789-61-7
- PubChem CID: 3000603
- IUPAC: 2-amino-9-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purine-6-thione
- INCHI Name: InChI=1S/C10H13N5O3S/c11-10-13-8-7(9(19)14-10)12-3-15(8)6-1-4(17)5(2-16)18-6/h3-6,16-17H,1-2H2,(H3,11,13,14,19)/t4-,5+,6+/m0/s1
- INCHI Key: SCVJRXQHFJXZFZ-KVQBGUIXSA-N
- Molecular Weight: 283.31
- Canonical SMILES: C1C(C(OC1N2C=NC3=C2NC(=NC3=S)N)CO)O
- Isomeric SMILES: C1[C@@H]([C@H](O[C@H]1N2C=NC3=C2NC(=NC3=S)N)CO)O
- Molecular Formula: C10H13N5O3S
Compound Supplier
- Supplier Name: Selleck Chemicals
- Catalog #: S7757
- Lot #: N/A
Compound Characterization
- HRMS (ESI-TOF) m/z: (M+H)+ Calcd for C10H13N5O3S 284.08119; found 284.07903
Dose Response Curve
Dose response curve not available.
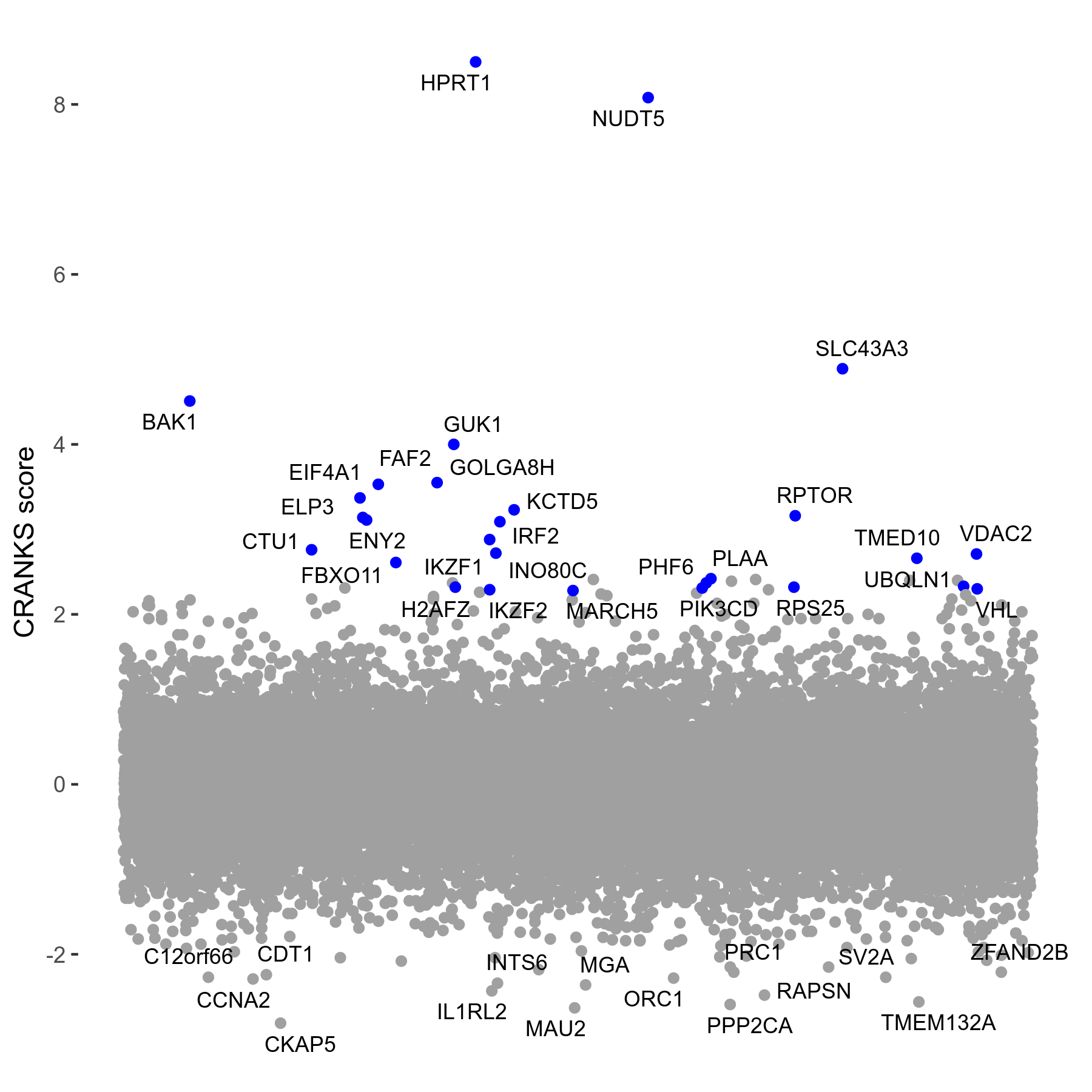
Screen Summary
- Round: 06
- Dose: 2µM
- Days of incubation: 8
- Doublings: 0.6
- Numbers of reads: 8752501
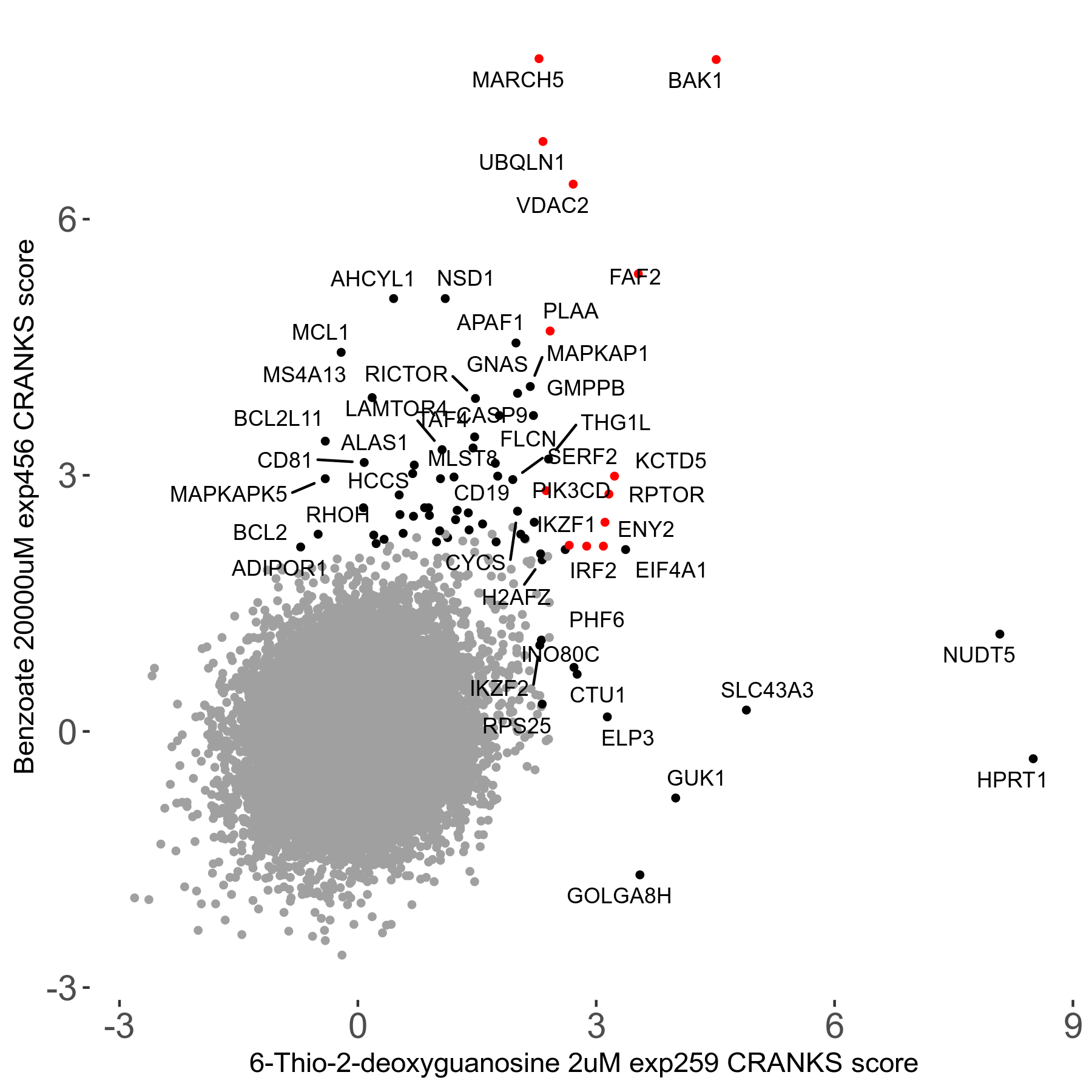
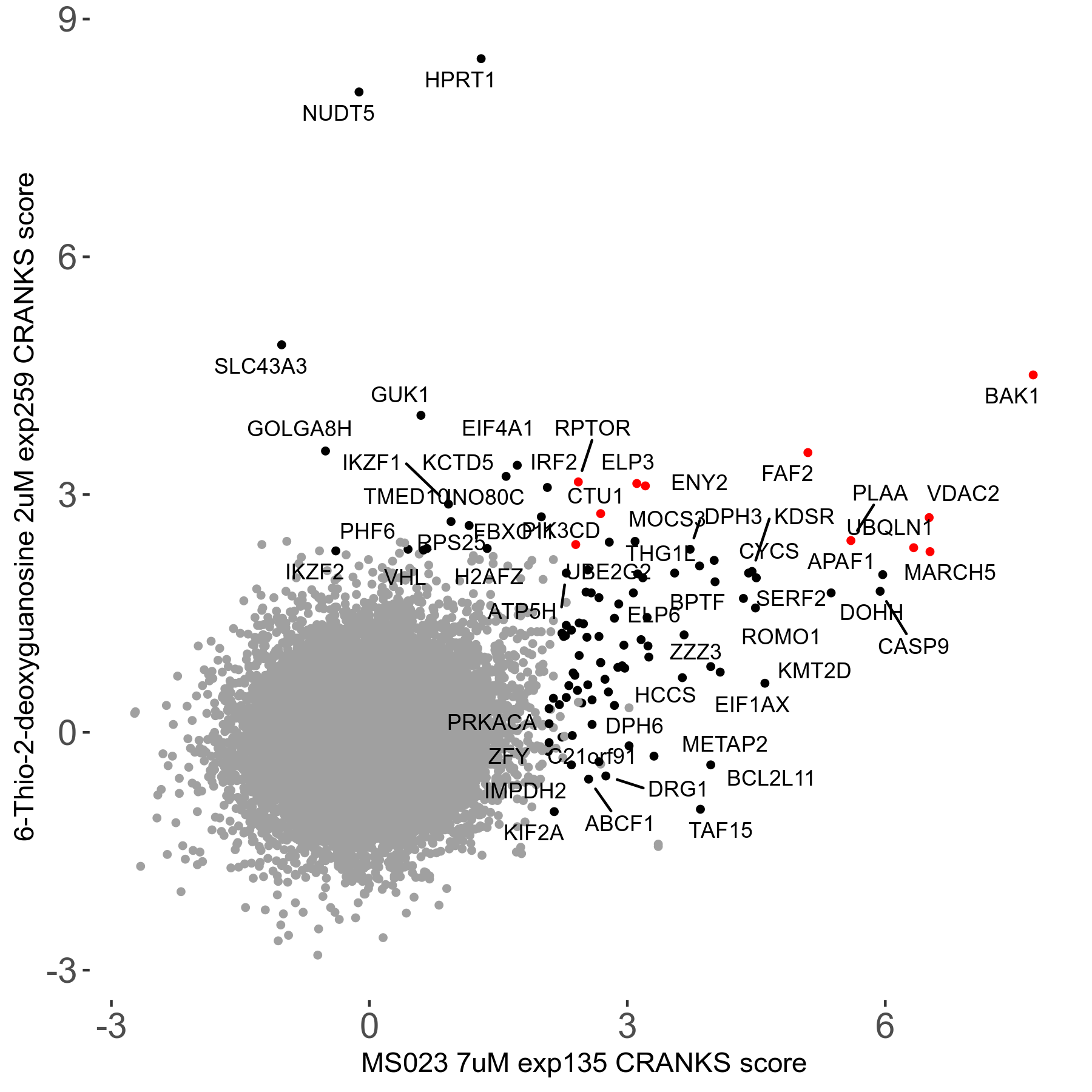
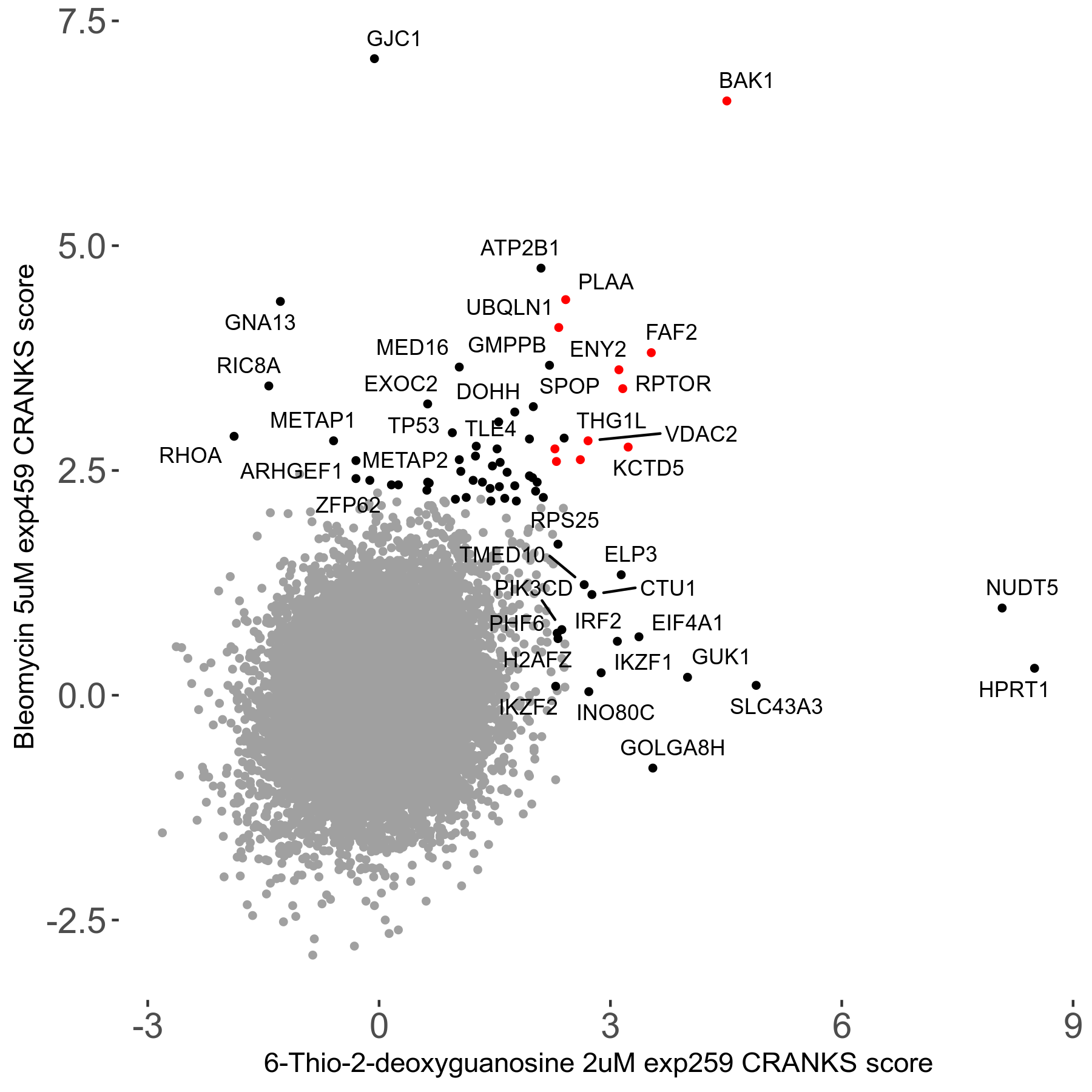
Screen Results
| Sensitive/Resistant hits (FDR<0.05) | CRANKS | Score Plot | Top 30 Genes | Screen Similarity | Top 30 Sensitive GO terms | Top 30 Resistant GO terms |
|---|---|---|---|---|---|---|
| 0/28 | Scores |